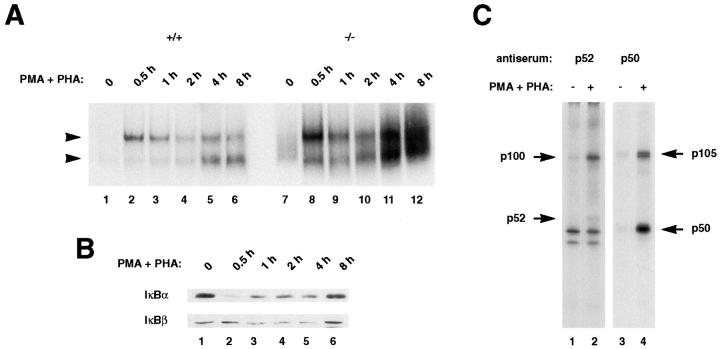
Figure 6.
Induction of κB-binding activity after stimulation of thymocytes. (A) Strong Rel/NF-κB activation in stimulated p100−/− thymocytes. Nuclear extracts (1.5 μg) from wild type (+/+, lanes 1–6) and p100−/− (−/−, lanes 7–12) thymocytes treated with PMA and PHA for the indicated periods were analyzed by EMSA. Two major bands are indicated by arrows. (Note: the exposure time of the autoradiogram is significantly shorter than that of Fig. 5 A). (B) IκBα is responsible for the rapid activation of the p50–RelA complexes in thymocytes. The amounts of the IκBα and IκBβ proteins in wild-type thymocytes stimulated with PMA and PHA for different periods were determined by Western blot analysis using 30 μg of cytoplasmic extracts. (C) The processing of the p105 precursor is enhanced by stimulation of thymocytes. Whole cell lysates from wild-type thymocytes labeled with [35S]methionine for 6 h in the absence (lanes 1 and 3) or presence (lanes 2 and 4) of PMA and PHA were incubated with a p52 antiserum (lanes 1 and 2) and the resulting supernatants were immunoprecipitated with a p50 antiserum (lanes 3 and 4). The exposure time of the autoradiogram of the immunoprecipitation with p52 is 2.5 times longer than that of p50, so as to verify the p100 and p52 proteins. Specific signals for the p100, p52, p105, and p50 proteins are indicated by arrows. The numbers of methionine residues contained in human p100, p52, murine p105, and p50 are 16, 10, 20, and 9, respectively (25, 66).