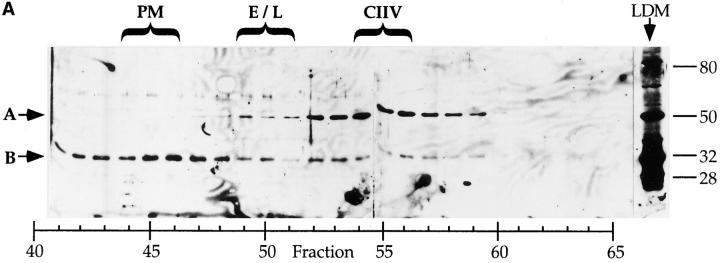
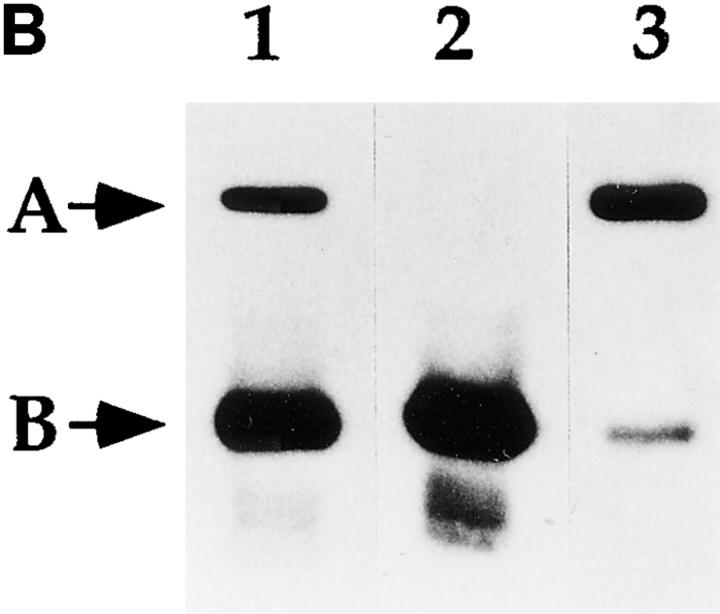
Figure 5.
Identification of a putative CIIV-marker protein that is immunologically related to Igα subunit of the BCR. (A) Identification of a 50-kD protein enriched in CIIV-containing FFE fractions. A20μWT cells were fractionated by FFE and the distribution of markers for plasma membrane (PM, unshifted class II), endosomes/lysosomes (E/L, β-Hexosaminidase), and CIIV (anodally shifted class II) were determined. The position and size in kilodaltons, of the molecular mass standards is indicated at the right. The distribution of the Igα subunit of the BCR was determined by Western blot analysis using a rabbit antiserum against Igα. The 32-kD Igα protein (indicated by B) was detected at the highest levels in PM-containing FFE fractions containing and to a lesser extent in fractions containing endosomes–lysosomes and CIIV. Additionally, a 50-kD protein (A) was selectively enriched in the CIIV-containing FFE fractions (fractions 52–59). The 50-kD protein is also detectable in the unfractionated LDM. Illustrated are results representative of five independent experiments. (B) The 50-kD putative CIIV-marker protein is immunologically related to the Igα subunit of the BCR. Preparative Western blots of A20 LDMs were probed with the rabbit anti-Igα antiserum and washed. The antibodies against Igα and the 50-kD protein were affinity purified by excising the regions of the blot containing the anti-Igα and anti-50-kD proteins (and bound antibodies) and then eluting the bound antibodies. The affinity-purified antibodies, as well as uncut antiserum, were then used to probe a Western blot of unfractionated LDM. The uncut serum (lane 1) recognized both the 32-kD Igα (arrow B) as well as the 50-kD protein (arrow A) in the LDM. Although the affinity-purified anti-Igα (lane 2) did recognize Igα, it failed to recognize the 50-kD protein present in the LDM. (In some experiments, the affinity-purified anti-Igα antibodies did demonstrate reactivity towards the 50-kD protein.) Importantly, antibodies affinity purified on the 50-kD protein (lane 3) recognized both the 32-kD Igα protein (arrow B) as well as the 50-kD protein (arrow A), indicating an immunological relationship between these two proteins. (Immunoreactivity of the affinity-purified anti-50-kD antibodies against Igα was observed in every experiment.) Illustrated are results representative of three independent experiments.