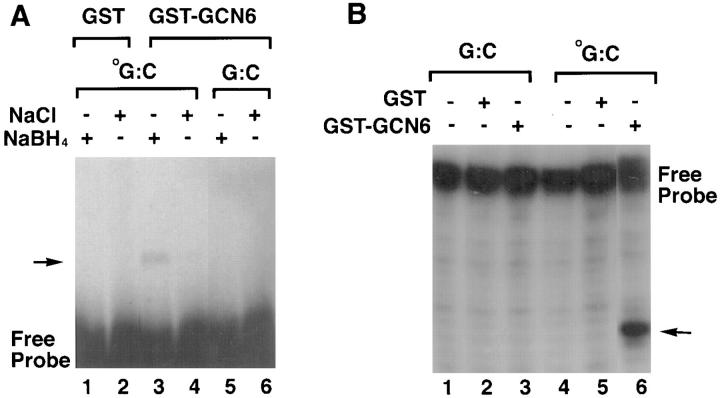
Figure 5.
Functional assays of the GST-GCN6 fusion protein. (A) Trapping of the Schiff base intermediate by the addition of sodium borohydride. Double-stranded, 3′ end–labeled oligonucleotides either with (oG:C, lanes 1–4) or without (G:C, lanes 5 and 6) an 8-oxoguanine at the center of the fragment were incubated with either with GST alone (lanes 1 and 2) or GST-GCN6 fusion protein (lanes 3–6) in the presence (lanes 2, 4, and 6) or absence (lanes 1, 3, and 5) of sodium borohydride. The reaction products were analyzed by 8% SDS-PAGE gel electrophoresis followed by autoradiography. The presence of a protein–DNA conjugate can be detected as a slow mobility band indicated by the arrow. (B) Cleavage of double-stranded oligonucleotides containing 8-oxoguanine. Double-stranded, 3′ end–labeled oligonucleotides with or without an 8-oxoguanine were incubated with GST alone (lanes 1 and 5) or GST-GCN6 fusion protein (lanes 3 and 6), and the reaction products analyzed by electrophoresis in a 12% polyacrylamide-7M urea gel followed by autoradiography.