Figure 1.
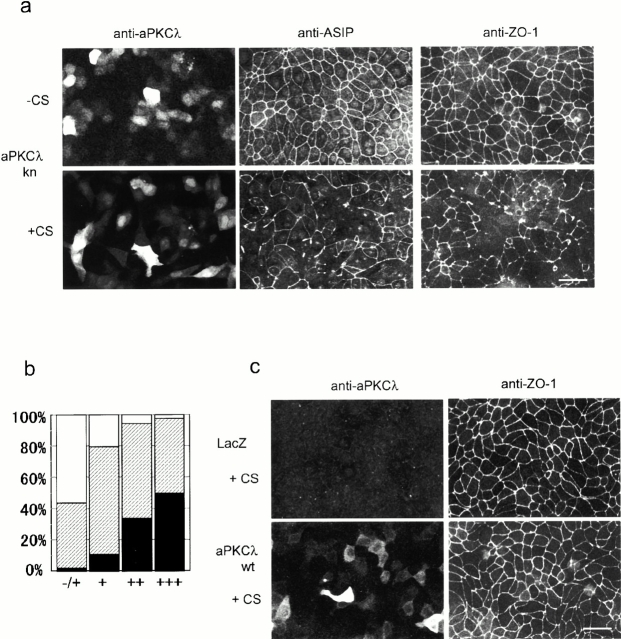
Overexpression of the kinase-deficient mutant of aPKCλ (aPKCkn) in confluent MDCK monolayers disrupts the junctional localization of ASIP and ZO-1 only when the cells are subjected to calcium switch. (a) Confluent MDCK cells seeded on cover slips were infected with adenovirus vector encoding aPKCλkn. 20 h after viral infection, the cells were subjected to calcium switch (+CS) or left untreated (−CS) (see Materials and Methods). After another 20 h, the cells were doubly labeled with anti–aPKCλ and anti–ASIP as indicated at the top (left and middle). Each photograph represents the projected view of optical sections (0.4 μm) obtained from the apical to basal membrane using a laser scanning confocal microscope (unless otherwise noted, the following data were obtained in the same way). Note that fluorescent signals for endogenous aPKCλ are not observed in the photographs because the anti–aPKCλ antibody used (λ2) is not sensitive enough to clearly detect the endogenous protein in MDCK II cells and because the exposure times were adjusted to cover the highly heterogenous level of aPKCλkn expression. (Right) Independent specimens stained by the anti–ZO-1 antibody. Bar, 25 μm. (b) Correlation between the expression level of aPKCλkn and the severity of ZO-1 mislocalization. Based on the fluorescent intensity of aPKCλ, cells were categorized as showing undetectable (−/+), low (+), medium (++), or high (+++) expression, and the ZO-1 staining pattern of the corresponding cell was estimated as complete (white), partially disappeared (hatched), or completely disappeared (black). Original statistical data are presented in Table . (c) LacZ- or aPKCλwt-expressing cells subjected to calcium switch were doubly stained as in a, with anti–aPKCλ and anti–ZO-1 antibodies. Bar, 25 μm.