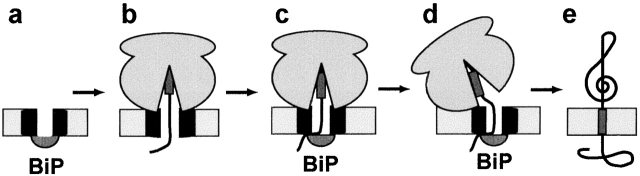
Figure 4.
Mechanism for maintaining the permeability barrier of the ER membrane during cotranslational membrane protein integration. (a) Prior to integration, the ribosome-free translocon is sealed on the lumenal side by BiP. (b) After SRP-dependent targeting of a ribosome-nascent chain complex to the translocon and translation to yield a nascent chain longer than 70 amino acids (Crowley et al., 1994), the ribosome–translocon seal is intact and the lumenal end of the pore is open (e.g., 111p-86). (c) After the TM sequence has been synthesized and is still near the peptidyltransferase center far inside the ribosome (Liao et al., 1997), the lumenal end of the translocon pore is closed by the action of BiP (111p-88, 111p-91). Although BiP is shown here physically plugging the pore, BiP may effect closure indirectly by binding to another protein(s) that physically closes the pore. At this point, the ribosome–translocon seal is still intact. (d) The ribosome–translocon seal is then broken, whereas the BiP-dependent seal at the other end of the pore remains intact (111p-93). Although the ribosome is depicted here as rotating relative to the translocon, the nature and magnitude of this structural change is not yet known. (e) After termination of translation, the TM sequence is integrated into the ER membrane.