Figure 3.
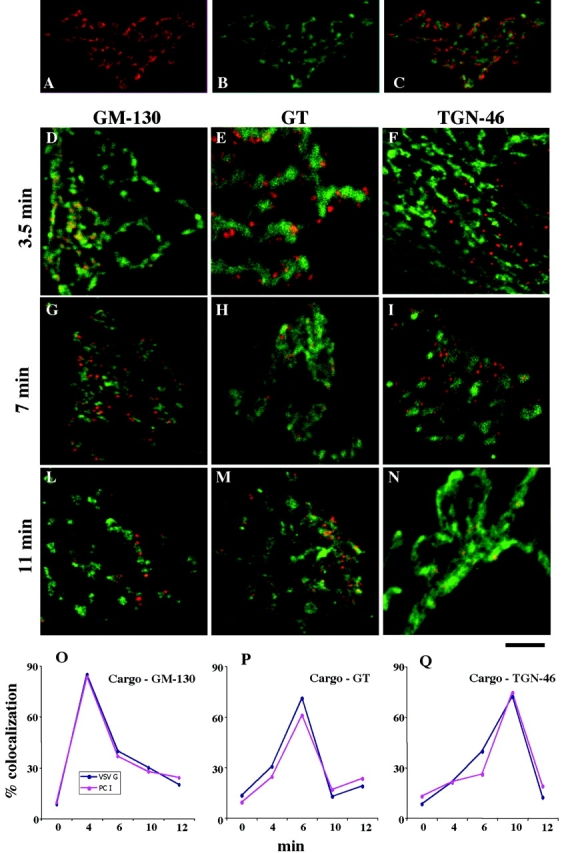
PC-I and VSVG move through the main Golgi subcompartments (cis-, medial-, and trans-TGN) at indistinguishable rates. Human fibroblasts were fixed at steady state (A–C) or subjected to the small-pulse protocol and fixed at various times after release of the 15°C block (D–N). (A–C) Golgi areas stained for GM130 (A, red) and TGN (B, green); the merged image is shown in C. Note that the patterns of two colors appear very similar in (A) and (B), but they clearly do not overlap (C). (D–N) Cells subjected to the small-pulse protocol were fixed at the times indicated in the figure after releasing the 15°C block and double labeled for VSVG and GM130 (D, G, and L), VSVG and GT (e, h, and m), or VSVG and TGN (f, i, and n). VSVG is red and GM130, GT, and TGN are green. At time 0, VSVG localized in peripheral spots (IC elements) and did not overlap with the Golgi markers (unpublished data). At 3.5 min, VSVG colocalized with GM130 (D) but not with GT (E) and TGN46 (F). Later (7 min), VSVG lost colocalization with GM130 (G) acquired colocalization with GT (H), and did not colocalize with TGN46 (I). Finally (11 min), VSVG lost colocalization with GM130 (L) and GT (M) and acquired colocalization with TGN46 (N). Identical results were obtained by labeling PC-I instead of VSVG (see below), and the two cargoes colocalized perfectly (unpublished data). (O–Q) Quantification and time course of the passage of VSVG and PC-I through the main Golgi subcompartments. The localization of cargoes in each subcompartment was assessed by measuring the degree of overlap of each cargo with the marker of each subcompartment (GM130, GT, and TGN) (see Materials and methods), and is expressed as the percentage of colocalization (percentage of cargo-containing pixels which also contain the appropriate Golgi marker). It is apparent that the two cargoes move together. Each value represents the average of eight to fourteen independent measurements from at least three different experiments. The SDs did not exceed 15% of the mean. Bar: (A–C) 12 μm; (D, F–I, L, and M) 10 μm; (E and H) μm 7,5; (N) 5 μm.
