Figure 6.
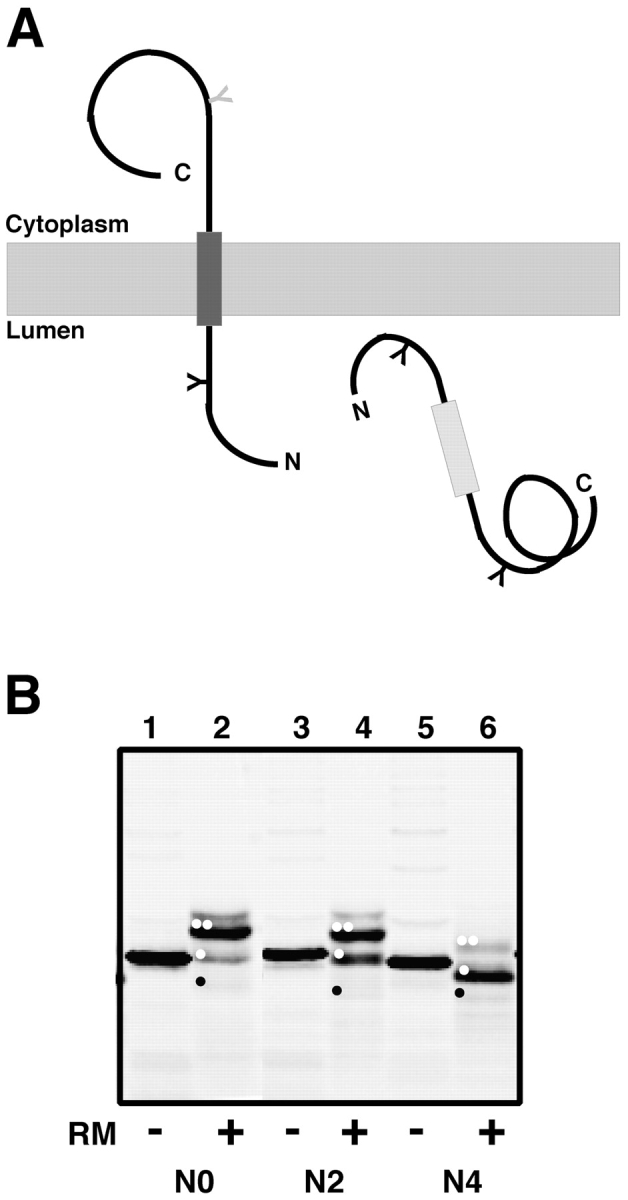
Conversion of the pPL-sK secretory protein into a membrane protein. (A) By introducing an NST sequence into the nascent chain behind the putative TM sequence, the extent of nascent chain integration into the ER membrane can be assessed (Sääf et al., 1998). If the potential TM segment does not function as a stop-transfer sequence (light gray rectangle), the entire protein will be translocated into the ER lumen and will be glycosylated at two sites. But if the TM segment is inserted into the bilayer (dark gray rectangle), only one of its glycosylation sites will be modified (black Y). (B) Full-length polypeptides containing zero (pPL-sK[NKT], which is the same as pPL-sK[N0]), two (pPL-sK[N2]), or four (pPL-sK[N4]) leucine substitutions in addition to an extra glycosylation site in the COOH-terminal portion of the protein were translated in reticulocyte lysate in the absence (−) or presence (+) of rough microsomes (RM). Signal sequence–cleaved diglycosylated, monoglycosylated, and nonglycosylated molecules are indicated by two white dots, one white dot, and one black dot, respectively. Very little cleaved, nonglycosylated product is seen in this experiment (black dot), but translation of the same construct lacking both glycosylation sites confirms the identity of this band (not depicted).
