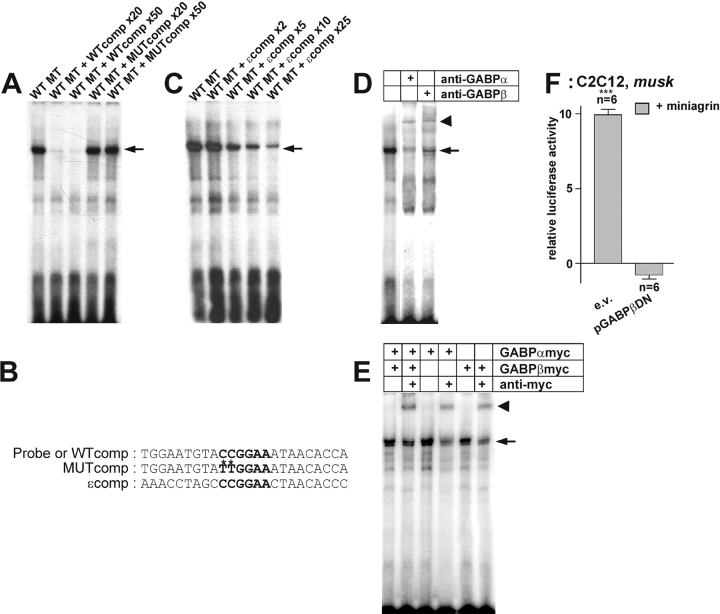
Figure 4.
GABP binds to the N-box of a nsk2/musk promoter fragment and activates it. (A) Binding activity of wild-type nuclear extracts to the N-box present in the probe. The specificity of the signal is eliminated by competition with an unlabeled wild-type probe (WTcomp), whereas an unlabeled mutant competitor (MUTcomp) leaves the signal unaffected. (B) Sequences of the oligonucleotides used as probes or competitors. (C) Unlabeled oligonucleotide containing the N-box motif of the AChRɛ subunit promoter (ɛ-comp) competes with N-box of musk for binding of nuclear extract. (D) The N-box binding activity produced by nuclear extracts from C2C12 cells (lane 1) is supershifted (bold arrow) by antibodies directed against GABPα (lane 2) and GABPβ (lane 3). (E) Nuclear extracts of transiently transfected HEK293 cells expressing GABPα-myc and GABPβ-myc (lanes 1 and 2) or only GABPα-myc (lanes 3 and 4) or GABPβ-myc (lanes 5 and 6) possess an N-box binding activity (arrow), which is supershifted (bold arrow) in the presence of an anti-myc antibody (Ab, lanes 2, 4, and 6). The second band seen in the shift may originate from binding activity specific for HEK293 cells. (F) Agrin-induced activation of the 2.1-kb nsk2/musk promoter fragment in C2C12 myotubes is blocked by overexpression of GABPβDN. ***P < 0.001 in two-tailed t test. Bars ± SEM. Note that for better resolution of the agrin-specific induction, luciferase activities in parallel control cultures (not depicted) are subtracted.