Figure 1.
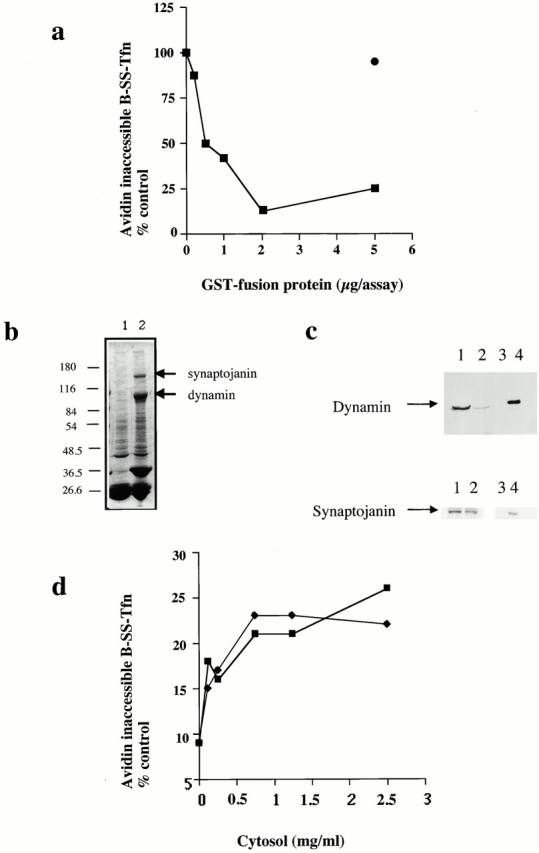
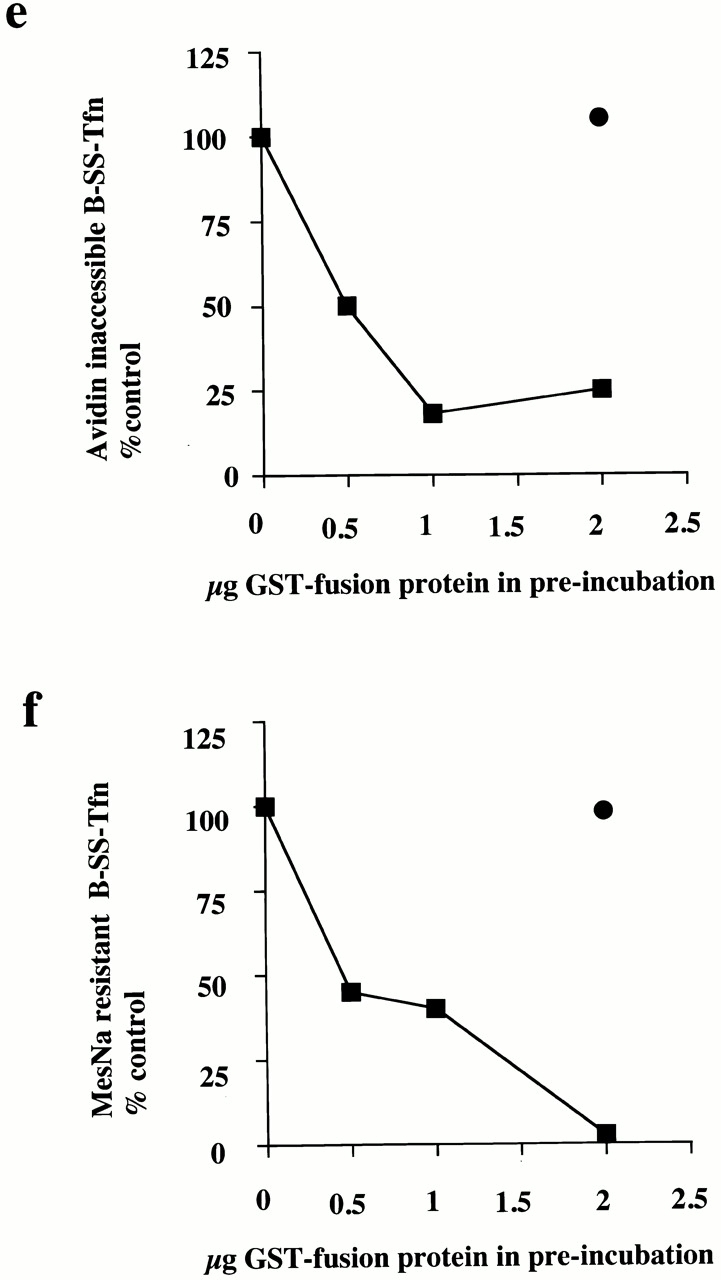
The SH3 domain of amphiphysin inhibits ligand sequestration and internalization in permeabilized A431 cells. (a) The sequestration of B-SS-Tfn into deeply invaginated coated pits and its internalization into coated vesicles was measured using the avidin internalization assay in the presence of the indicated concentrations of GST fusion proteins containing GST alone (filled circles), or the mutant SH3 domain of amphiphysin GST–amph2 SH3D36R (filled squares). Results are from a typical experiment where each assay point is the mean of duplicates which differed by <10%. (b) Bovine brain cytosol (500 μl of 10 mg/ml) was treated with glutathione-agarose alone or coupled to GST–amph2 SH3D36R for 2 h at 4°C. The beads were pelleted and washed three times in PBS before being electrophoresed on a 10% SDS-PAGE and Coomassie stained. Lane 1, GSH beads alone; lane 2, GST–amph2 SH3D36R beads. (c) Western blot of cytosol after treatment with GST–amph2 SH3D36R. Cytosol was treated as described in b and equivalent volumes of supernatant (lanes 1 and 2) or beads (lanes 3 and 4) were probed on Western blots using either antidynamin or antisynaptojanin antibodies. (d) The avidin inaccessibility assay was carried out in the presence of increasing concentrations of bovine brain cytosol which had been treated with GSH beads alone (filled squares) or with GSH beads coupled to GST–amph2 SH3D36R (filled diamonds). (e and f) Permeabilized cells were preincubated with GST (filled circles) or GST–amph2 SH3D36R (filled squares) at the indicated concentrations for 5 min at 30°C. The membranes were then pelleted by centrifugation and assayed either for (e) avidin inaccessibility or (f) MesNa resistance of B-SS-Tfn in the presence of cytosol (2.5 mg/ml) and ATP.
