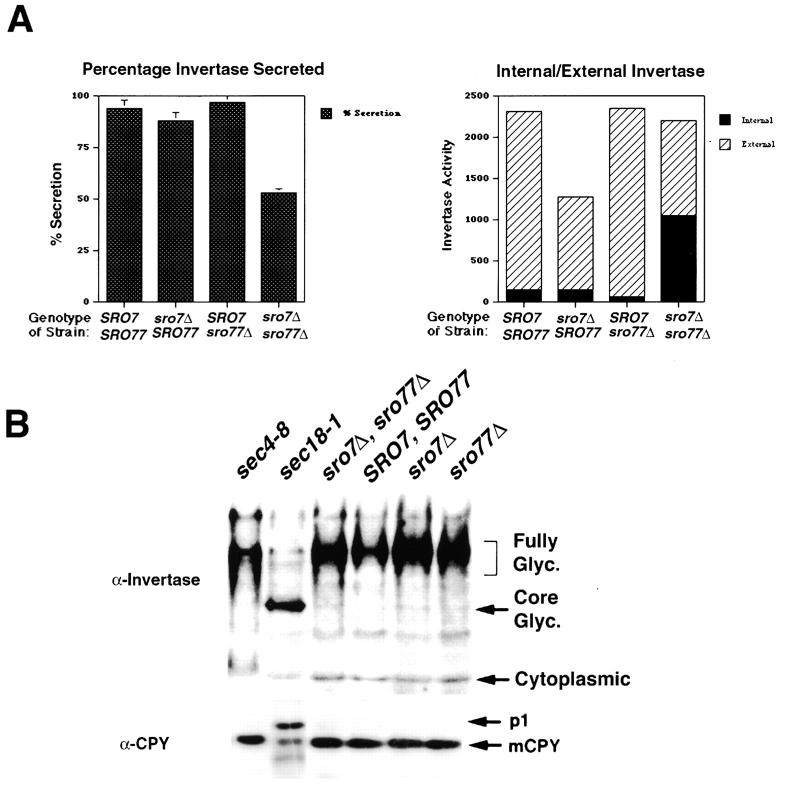
Figure 3.
The sro7Δ, sro77Δ mutant has a pronounced defect in Golgi-to-cell surface transport. (A) Invertase secretion is deficient in the sro7Δ, sro77Δ mutant strain. Invertase secretion assays were performed after a temperature shift on several tetratype tetrads and a representative example is shown. The left frame shows a comparison of the percentage of invertase secreted into the periplasm after a 3-h shift to the restrictive temperature (19°C). The right frame shows the partitioning of invertase activity in the internal (intracellular) and external (periplasmic) fractions of these cells. All samples were measured in duplicate and the SD determined from two separate experiments. (B) The invertase that accumulates in sro7Δ, sro77Δ cells is fully glycosylated and transport of CPY to the vacuole is unaffected. Wild-type, single-disruptant, and double-disruptant strains, in addition to sec4-8 and sec18-1 strains, were grown and shifted as in A, except that sec4-8 and sec18-1 strains were grown for 2 h at 37°C before lysis. Equivalent amounts of each strain were lysed with glass beads, boiled in SDS sample buffer, subjected to SDS-PAGE, transferred to nitrocellulose, and probed with antibodies raised against invertase (top) or CPY (bottom). Mutants that block secretion after exit from the Golgi apparatus, such as sec4-8, accumulate internal pools of fully glycosylated invertase, whereas mutants that block at earlier stages, such as sec18-1, accumulate the core-glycosylated or ER-modified form of invertase. Likewise post-Golgi sec mutants do not affect CPY transport and maturation (and therefore show only the mature, mCPY form), unlike mutants that block earlier in the pathway such as sec18-1, which accumulates the core-glycosylated, p1 form of CPY. The forms of invertase and CPY found in the sro7Δ, sro77Δ cells suggest it is primarily affecting Golgi-to-cell surface transport.