Figure 7.
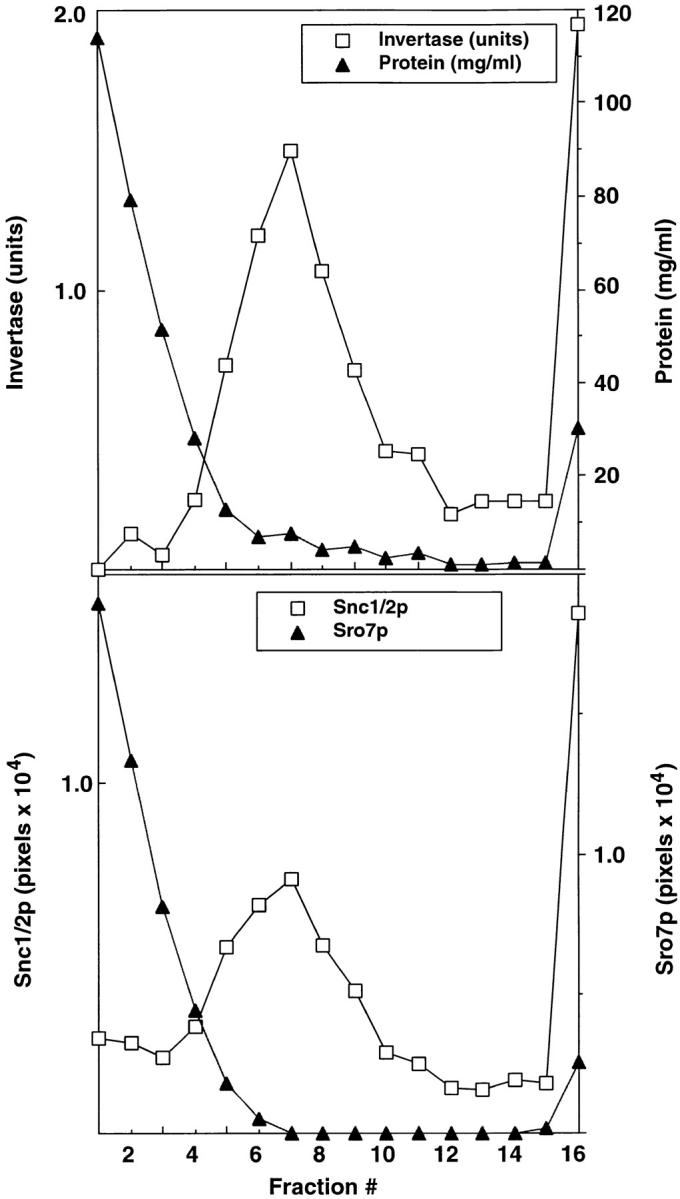
Sro7p is not present on post-Golgi secretory vesicles. Cells from a BY29 (sec1-1, ura3-52) strain were shifted to 37°C in YP medium with 0.1% glucose for 2 h to accumulate post-Golgi vesicles and induce expression of invertase. Spheroplasts were prepared from these cells that were lysed osmotically by gentle resuspension in lysis buffer containing 0.8 M sorbitol. A 100,000 g membrane fraction (P3) enriched in post-Golgi vesicles was prepared from a 10,000 g supernatant fraction by differential centrifugation as described previously (Walworth and Novick 1987). The P3 vesicle fraction was resuspended in lysis buffer and layered onto a 20–40% sorbitol velocity gradient as described previously (Brennwald et al. 1994). The top shows the fractionation profile of latent invertase (a marker for secretory vesicles) compared with the distribution of total protein in the gradient. Invertase activity is expressed as micromoles of glucose per minute per fraction. Protein concentrations were determined according to Bradford 1976. The bottom shows the fractionation of Sro7p compared with Snc1/2, a marker for post-Golgi vesicles (Protopopov et al. 1993). The presence of Sro7p and Snc1/2 in the gradients was determined by immunoblotting followed by quantitation on a STORM PhosphorImager using ImageQuant software. Similar results were obtained using a P3 fraction prepared from a sec6-4 strain.
