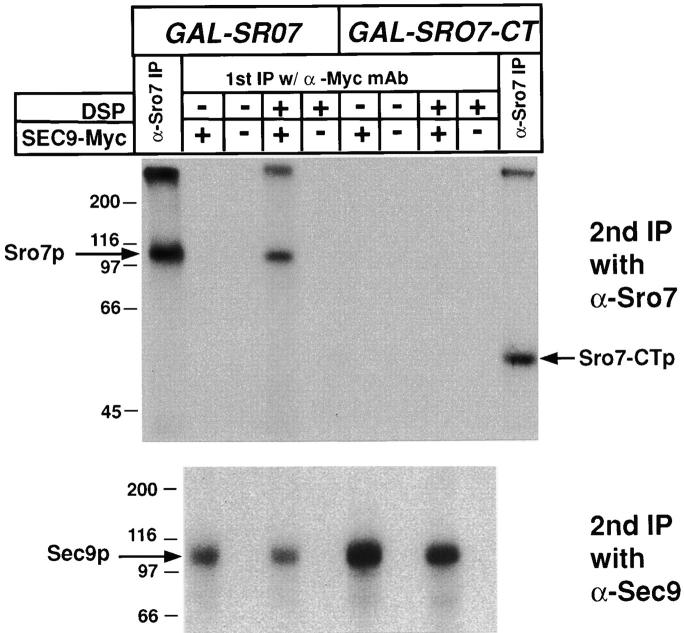
Figure 8.
Full-length Sro7p, but not the COOH-terminal half of Sro7p, can be directly cross-linked to Sec9p in yeast lysates. Coprecipitation analysis was performed on radiolabeled yeast strains transformed with either myc-tagged YEpSEC9-myc plasmid (pB37) or, as a control, an otherwise identical untagged YEpSEC9 plasmid (pB37) in strains which contained a construct overexpressing either full-length Sro7 (pB363) or CT Sro7 (pB367) under control of the inducible GAL1 promoter. All strains were induced in galactose-containing media for 2 h before 1 h labeling of cells with 35S-Express label in galactose-containing media. The cells were spheroplasted and lysed osmotically in PBS. Lysates were immediately subjected to treatment with the protein cleavable cross-linking agent (DSP) dissolved in DMSO or a DMSO control for 20 min on ice and subsequently boiled in 1% SDS buffer before dilution with IP buffer. Association because of cross-linking was monitored by a two-step immunoprecipitation protocol with the first immunoprecipitation being with the α-myc or directly with α-Sro7p antibodies (α-Sro7 IP). After washing, samples were boiled in 1% SDS/0.1 M DTT (to cleave the cross-linker), diluted with IP buffer, and subjected to a second round of immunoprecipitation with either α-Sro7p (top) or α-Sec9p (bottom) polyclonal antibodies. Samples were boiled in sample buffer, resolved by SDS-PAGE, dried, and exposed to film. The expression from the GAL1-SRO7 and GAL1-SRO7-CT constructs were similar (compare first and last lanes, marked α-Sro7 IP). A cross-linker and myc tag–dependent interaction is apparent between full-length Sro7p and Sec9p, strongly suggesting that these proteins are directly associated with each other in vivo. In contrast, the COOH-terminal domain does not show a detectable cross-linking in this assay.