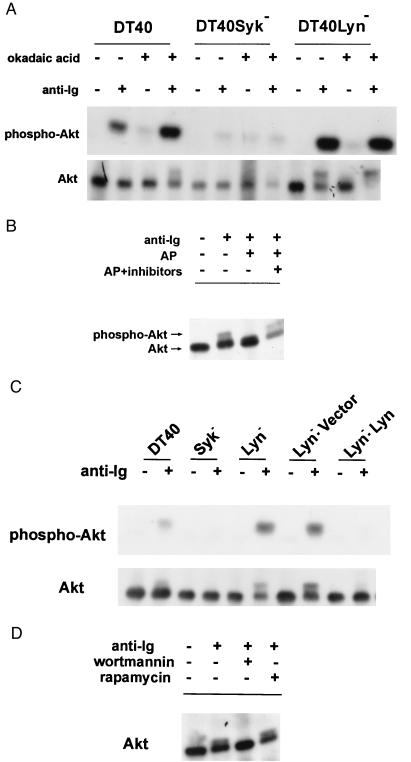
Figure 2.
BCR-mediated activation of Akt/PKB is Syk-dependent but inhibited by Lyn. (A) Okadaic acid-sensitive phosphatase(s) and Lyn inhibit anti-Ig induced phosphorylation of Akt. DT40, DT40Syk−, and DT40Lyn− cells were incubated in the presence or absence of okadaic acid before stimulation with M4 mAb. The phosphorylation state of Akt was analyzed by immunoblotting with anti-Akt (Lower) and immunoreactivity with anti-phosphoSer-473-Akt antibody (Upper). (B) Cell lysates were subjected to immunoprecipitation with anti-Akt Ab. The immune complexes were treated with alkaline phosphatase in the presence or absence of phosphatase inhibitors. The immune complexes were immunoblotted with anti-Akt. (C) Expression of human Lyn inhibited anti-Ig induced activation of Akt in DT40Lyn− cells. DT40, DT40Lyn−, and DT40Lyn− cells stably transfected with empty pApuro vector or a recombinant vector encoding human Lyn were stimulated with M4. Phosphorylation of Akt was measured as described in A (Upper, immunoblotted with anti-Akt; Lower, immunoblotted with anti-phosphoSer-473–Akt). (D) Cells were treated with 50 nM wortmannin or 10 nM rapamycin for 15 minutes before stimulation with M4 Ab. The level of phosphorylated Akt was measured as described in B.