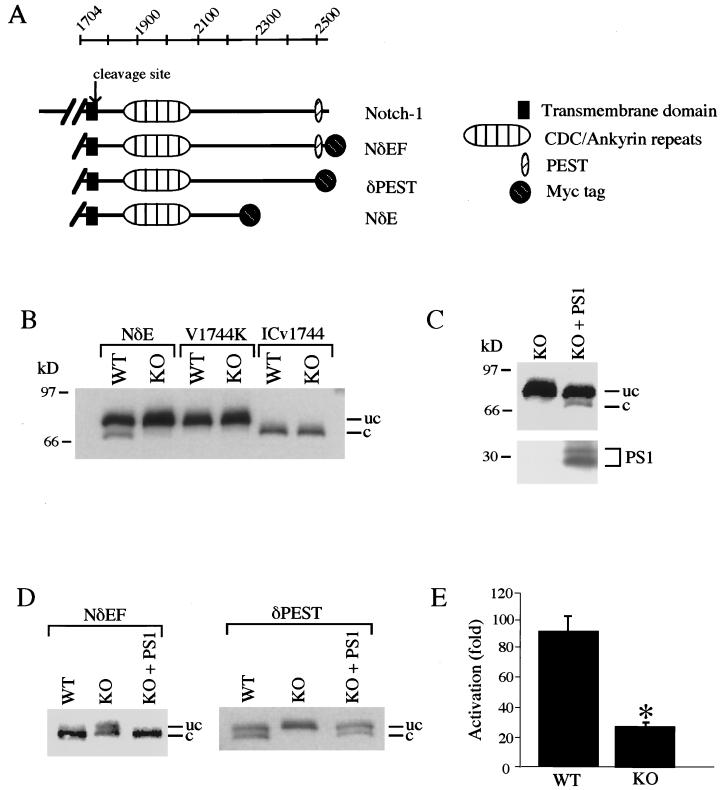
Figure 1.
PS1 induces proteolytic release of the Notch-1 intracellular domain. (A) Notch-1 constructs utilized in assays of proteolytic cleavage. (B) Release of the Notch-1 intracellular domain is markedly reduced in PS1-KO cells. PS1-wt (WT) and PS1-KO (KO) cells were transfected with Myc epitope-tagged NδE, NδE(V1744K), or ICv1744, and immunoblot analysis was performed with monoclonal anti-Myc. Note that the cleaved form of NδE is generated in PS1-wt cells but not in PS1-KO cells. The NδE mutation V1744K prevents cleavage, and ICv1744 is truncated at the cleavage site (3). The uncleaved (uc) and cleaved (c) NδE proteins are indicated. Immunoreactivity was absent in immunoblots of untransfected cell lysates. (C) PS1 restores Notch-1 proteolytic release in PS1-KO cells. (Upper) NδE immunoblot. (Lower) PS1 immunoblot showing PS1 N-terminal fragments. (D) The NδEF construct containing the full-length Notch-1-intracellular domain is cleaved completely, and cleavage depends on PS1 and the presence of a PEST motif. Note that PS1-wt cells expressing NδEF show only the cleaved protein, whereas PS1-wt cells expressing the PEST deletion mutant show similar amounts of cleaved and uncleaved proteins. Shown are NδE immunoblots of PS1-wt and PS1-KO cells transfected with Myc-tagged NδEF or δPEST constructs. (E) Transcriptional activation of the HES-1 promoter is potentiated by PS1. NδEF-induced transcriptional activation of the HES-1 promoter was markedly reduced in PS1-KO cells. PS1-wt and PS1-KO cells were cotransfected with NδEF and HES-1-luciferase together with a β-galactosidase expression plasmid (5). Values represent the ratio of normalized luciferase activity to normalized activity determined with the luciferase plasmid alone and represent the mean ± SEM, n = 9. ∗, P < 0.01 relative to PS1-wt by ANOVA with post-hoc Student–Newman–Keuls test.