Figure 1.
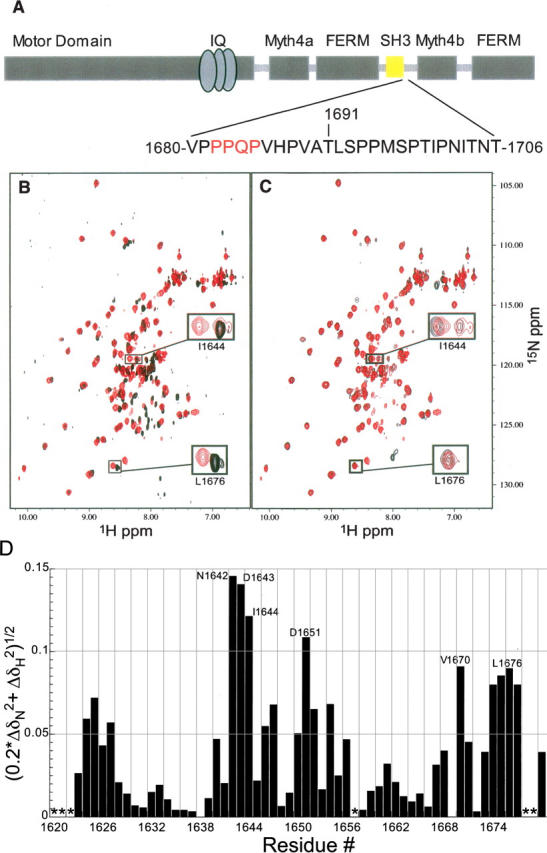
The DdM7 SH3 domain interacts with an adjacent proline-rich region. (A) Architecture of DdM7 with the SH3 domain highlighted in yellow. The sequence immediately following the SH3 domain spanning residues V1680–T1706 is also displayed with the interacting residues in red. (B) Superimposed [1H,15N] HSQC spectrum of the construct containing only the SH3 domain (A1620–V1680) (black) with that of a longer construct containing the adjacent proline-rich region (A1620–T1706) (red). This comparison reveals that certain resonance cross-peaks in the SH3 domain shift due to the presence of the proline-rich extension. (C) Superimposed [1H,15N] HSQC spectra of A1620–T1706 (red) and A1620–H1687 (black) reveal that P1681–H1687 contains the residues responsible for the chemical shift perturbations observed in the SH3 domain. (D) Amide chemical shift perturbation analysis reveals the residues of the SH3 domain most affected by the presence of the adjacent C-terminal region. For this analysis, Equation 1 was used to compare the amide chemical shift values of the SH3 in the shorter construct spanning A1620–V1680 with those in the construct spanning A1620–T1706. Asterisks represent residues lacking chemical shift assignments.
