Figure 6.
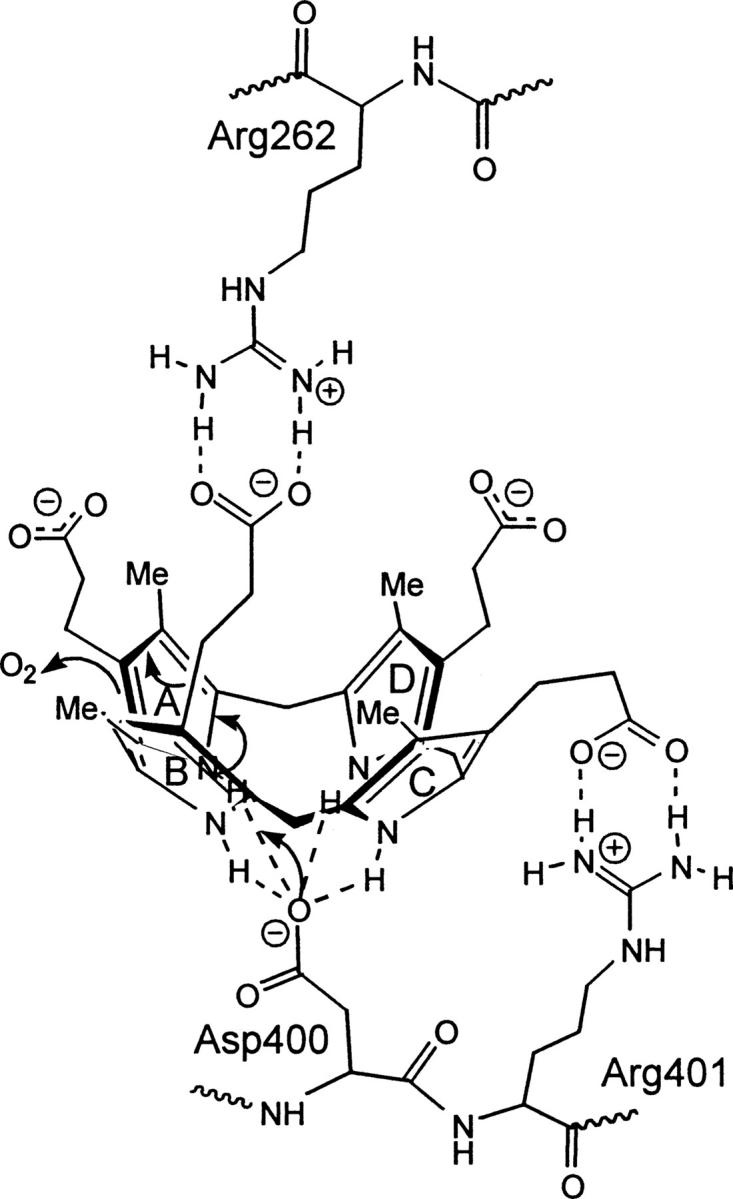
Proposed CPO active site interactions of copro'gen-III with arginine 262, aspartate 400, and arginine 401. Arginine 262 forms key interactions with the propionate moiety on ring B and arginine 401 forms key interactions with the propionate on ring C of copro'gen-III. The anionic residue, aspartate 400, is proposed to play a role in catalysis by coordinating to the central pyrrolic NH groups to stabilize a bowl conformation of copro'gen-III, thus leading to initial deprotonation of the A ring and subsequent oxidative decarboxylation. A speculated binding partner or role for ring D has not yet been determined.
