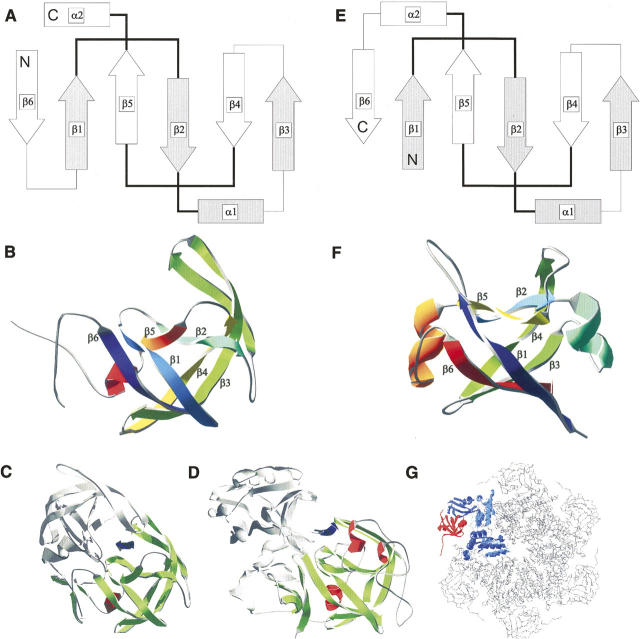
Figure 1.
Structural comparison of double-ψ barrel proteins and aspartic proteases. Topology and structure of HIV-1 protease, pepsin, and Cdc48 N-terminal domain-like double-ψ barrels. Topology diagrams of (A) HIV-1 protease and a (E) double-ψ barrel. β-strands are represented as arrows, α-helices as bars, and loops as lines. Pseudo-symmetric units are depicted as filled and empty forms. The “ψ”-forming loops are in bold. The secondary structure elements are totally interleaved—elements of one symmetric unit make only contacts to elements of the other unit. Structures shown as ribbon diagrams. Order of secondary structure elements is color-coded from blue to red: (B) monomer of HIV-1 protease (5hvp) and (F) N-terminal double-ψ barrel domain of VAT (1cz4) from Thermoplasma acidophilum. (Bottom) Structural context of the domains: (C) One monomer of the HIV-1 protease dimer is colored and the substrate binding site is indicated by an arrow between both domains. (D) One domain of pepsin is colored and the substrate binding site is indicated by an arrow between both domains. (G) Structure of p97 (1r7r) representing the structural context of Cdc48 N-terminal domain-like double-ψ barrels. One monomer of the hexamer is colored with the N-terminal double-ψ barrel domain in red.