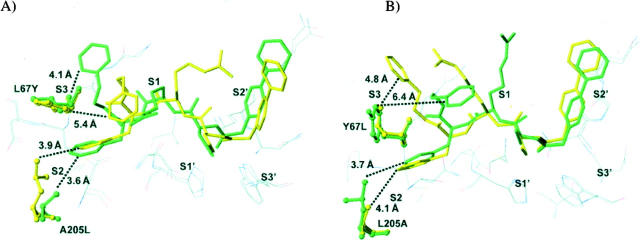
Figure 2.
Binding mode of N-(4-biphenylacetyl)-S-methylcysteine-(D)-Arg-Phe-β-phenethylamide (Cat L inh. 7) with (A) cathepsin L and its Leu67Tyr/Ala205Leu variant, and with (B) cathepsin K and its Tyr67Leu/Leu205Ala variant. Wild-type (green) and mutant (yellow) binding modes are superimposed. (Dashed lines) The shortest distances between the P3 phenylethyl moiety (phenyl ring) and the S3 subsite, and the phenyl ring of Phe at P2 and the S2 subsite, respectively. The inhibitor is represented in stick mode and in ball-and-stick mode for the mutated protein side chains. Other selected nearby protein residues are show as thin lines.