Figure 1.
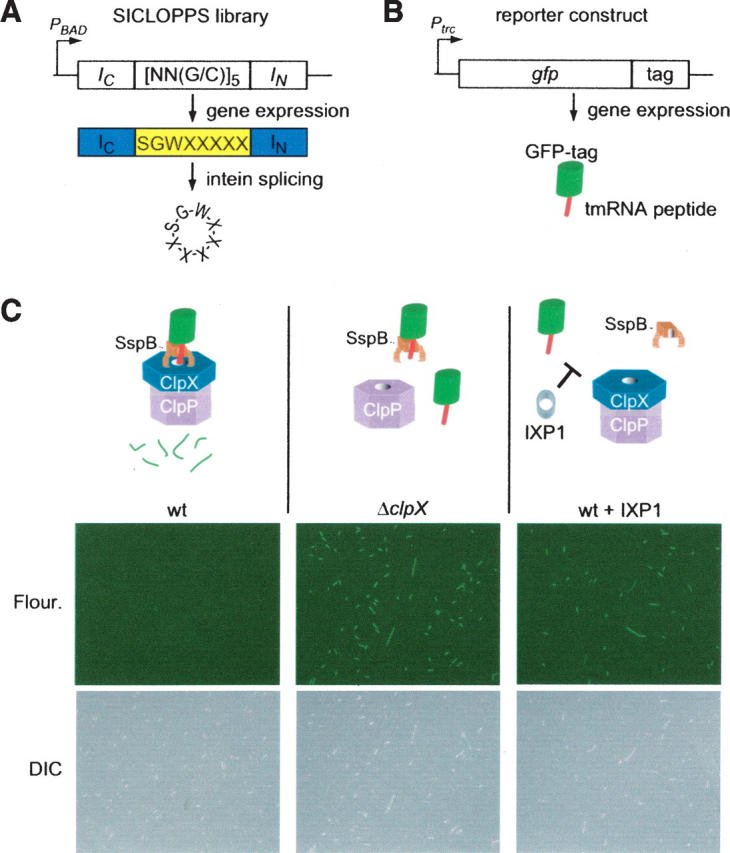
Selection for cyclic peptide inhibitors of ClpXP. (A) Schematic diagram of SICLOPPS library construction. Three fixed and five randomized codons were cloned in the coding region between the intein IC and IN domains. When this gene is expressed, the IC and IN domains promote circular ligation of the intervening peptide, resulting in 8-mer cyclic peptides with five random amino acids. (B) Schematic diagram of the GFP-tag reporter. The tmRNA peptide tag sequence was encoded at the 3′-end of the gfp gene under control of an IPTG-inducible promoter. All GFP produced from this gene will have the tmRNA peptide tag at the C terminus. (C) Result of expression of the GFP-tag reporter and inhibitory cyclic peptides in E. coli. Production of GFP-tag was induced in wild-type E. coli (wt), a strain lacking the clpX gene (ΔclpX), and a strain that was also producing the IXP1 cyclic peptide; the cells were imaged by immunofluorescence (fluor.) to see fluorescent cells and differential interference contrast microscopy (DIC) to see all cells. In wild type, SspB binds to the tmRNA peptide at the C terminus of GFP-tag and tethers the protein to the ClpXP protease, resulting in rapid degradation and no fluorescent cells. In the ΔclpX strain, the absence of active ClpXP protease results in stabilization of GFP-tag and highly fluorescent cells. In wild-type cells producing IXP1, the cyclic peptide inhibits degradation of GFP-tag, resulting in fluorescent cells. DIC images show that the ΔclpX cells and wild-type cells producing IXP1 are also slightly filamentous.
