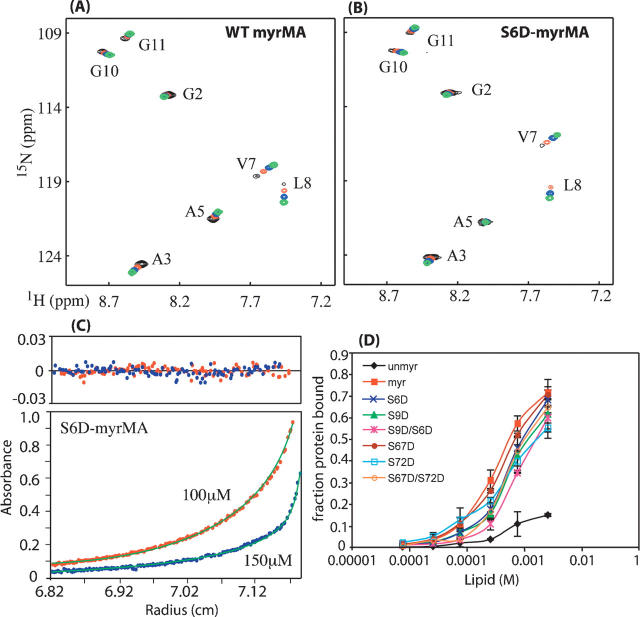
Figure 1.
Overlay of two-dimensional 1H-15N HSQC spectra for WT (A) and S6D-myrMA (B) proteins collected at different concentrations (800–900 μM [black], 300–400 μM [red], 150 μM [blue], 50 μM [green]). Signals indicative of myristyl switch from sequestered form at low concentration to exposed form at high concentration shift similarly for WT and S6D-myrMA proteins. (C) Representative sedimentation profiles obtained for S6D-myrMA (100 μM [red], 150 μM [blue]). (D) Effect of lipid concentration on MA–lipid interaction. The apparent binding constant is ∼10−3 M. Data show that mutant HIV-1 myrMA proteins bound to liposomes with ∼1–2 lower affinity than native myrMA for most mutants except S6D/S9D, which showed ∼3 times weaker than native. Unmyristylated MA bound very weakly to liposomes.