Figure 2.
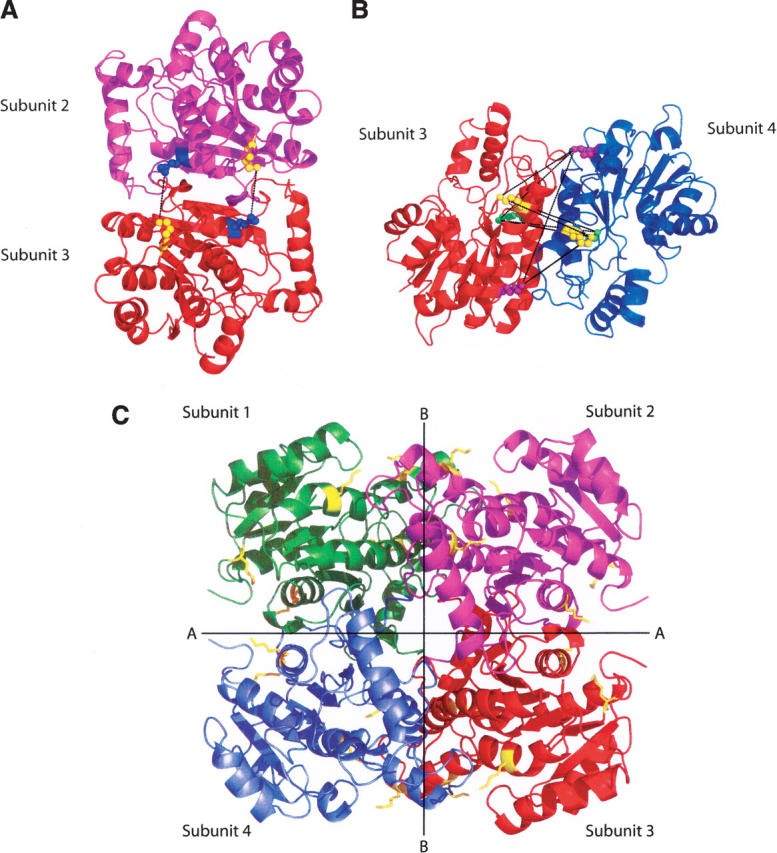
Interactions across the dimer–dimer interfaces in InhA. (A) Dimer formed by subunits 2 and 3 (1BVR.pdb), rotated 90° to clearly show the cross-links, in black, formed between Lys240 (blue spheres) and Lys8 (yellow spheres), across interface A. The two lysine pairs are separated by 9.86 Å and 9.89 Å, respectively. (B) Dimer formed by subunits 3 and 4 (1BVR.pdb), rotated to show all nine possible cross-links (in black) across interface B. The three lysines, 118, 132, and 165, in each monomer are highlighted in yellow, purple, and green. Distances between pairs of lysine residues from subunit 3 to subunit 4 range from 18.58 Å to 32.25 Å. In this ligand-bound structure, none of the lysine pairs are close enough to be cross-linked by BS3. (C) The InhA tetramer, taken from the structure of InhA bound to a C16 substrate and NAD+ (1BVR.pdb). The subunits are labeled 1–4, and the two distinct dimer–dimer interfaces, A and B, are shown. All lysine residues are shown in yellow.
