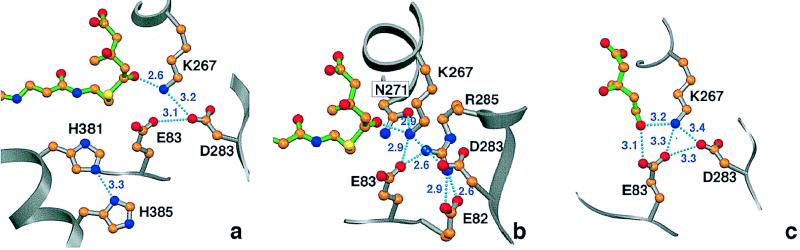
Figure 4.
Ball and stick diagrams of the HMG-CoA (a and b) and mevalonate (c) substrates in the active site of HMG-CoA reductase. The substrates are shown as stick models with C = orange, N = blue, O = red, and S = yellow. (a) Critical contacts with the enzyme (silver bonds) at the site of cleavage of HMG-CoA (green bonds). The thioester oxygen of the substrate points directly at Lys267, making a strong hydrogen bond with this newly identified catalytic residue. The contact between the catalytic His381 and His385, proposed to aid in the protonation of the CoAS− group, also is shown. (b) The hydrogen bonding network that holds Lys267 in place. Shown are the first shell of hydrogen bond contacts that involve primarily positive and negatively charged residues. (c) Critical contacts of the catalytic residues with the mevalonate substrate. In this ternary complex, the tetrahedral carbon (C-5) points its OH toward one of the Glu83 carboxylic oxygens, suggesting a change in the primary contact of that oxygen after isomerization. This also suggests that Glu83 may be involved in accepting a proton from mevaldyl-CoA, which should have the same tetrahedral configuration for C-5. This figure was prepared with setor (20).