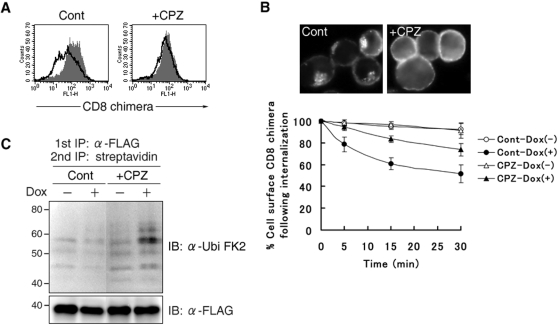
Figure 2. Accumulation of ubiquitinated surface CD8 chimera by inhibiting internalization.
(A) After being incubated with or without Dox for 3 hr, T-REx-c-MIR was further cultivated for 5 hr with chlorpromazine (10 µg/ml) (+CPZ) or water (Cont) in the presence or absence of Dox. At the end of incubation, the expression of surface CD8-B7-2-A2 on T-REx-c-MIR was examined by FACS. Data from cells incubated with Dox (open histograms) and cells incubated without Dox (shaded histograms) are shown. Data are representative of two independent experiments. (B) T-REx-c-MIR was treated as in A. Treated T-REx-c-MIR was incubated in the presence of FITC-conjugated CD8 Ab at 37°C for 10 min, and internalized CD8-B7-2-A2 was examined by fluorescence microscopy. For the quantitative analysis of internalization, surface CD8-B7-2-A2 of T-REx-c-MIR treated as above was labeled with anti-CD8 Ab, and cultivated at 37°C for the indicated times. The expression of remaining surface CD8-B7-2-A2 was examined by staining with PE-conjugated goat anti-mouse IgG. At each point, the percentage of remaining CD8-B7-2-A2 was calculated as in Figure 1F. (C) Surface molecules of T-REx-c-MIR were biotinylated first as in Figure 1C. After biotinylated T-REx-c-MIR was incubated with (+) or without (−) Dox for 3 hr, biotinylated T-REx-c-MIR was further cultivated with chlorpromazine (10 µg/ml) (+CPZ) or water (Cont) for 5 hr in the presence (+) or absence (−) of Dox. After cultivation, biotinylated CD8-B7-2-A2 was sequentially purified with anti-FLAG Ab and streptavidin-agarose. Each sample was probed with anti-ubiquitin Ab (upper panel) or anti-FLAG Ab (lower panel). Data are representative of two independent experiments.