Abstract
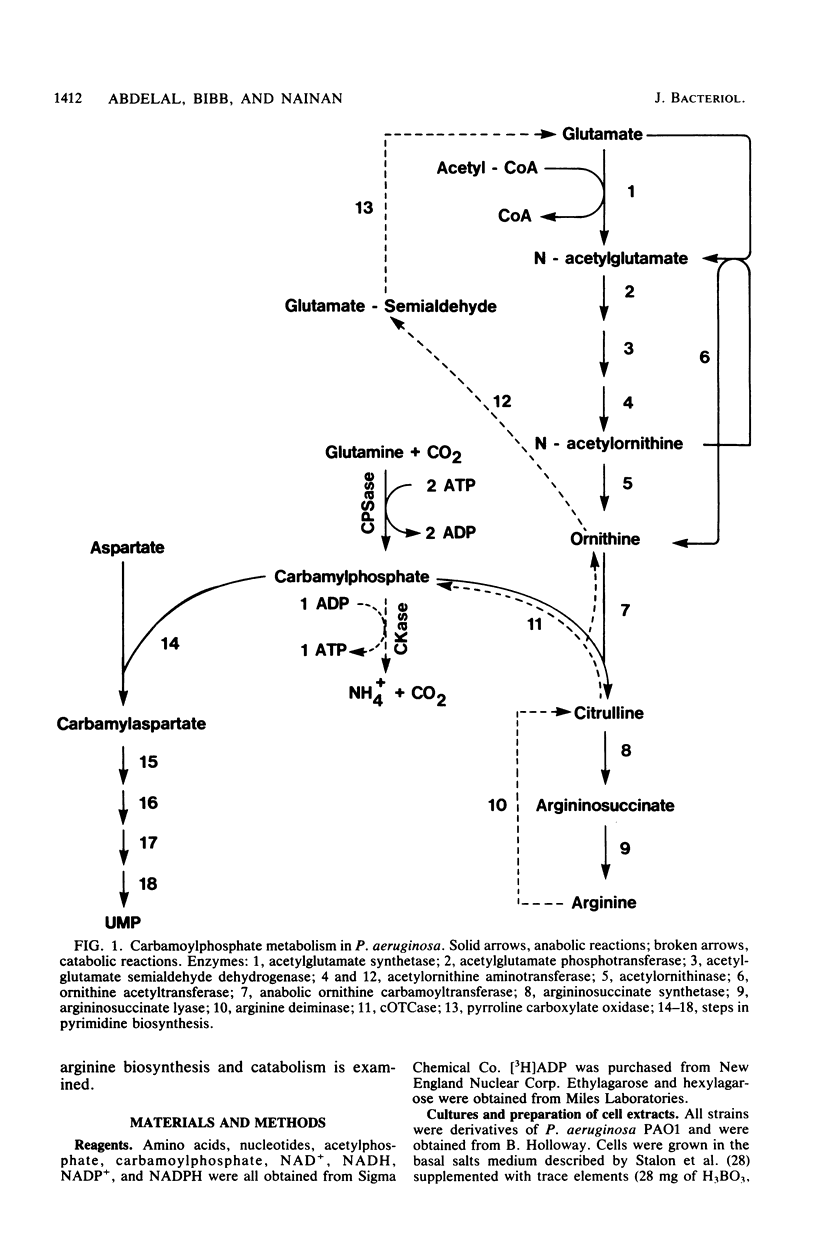
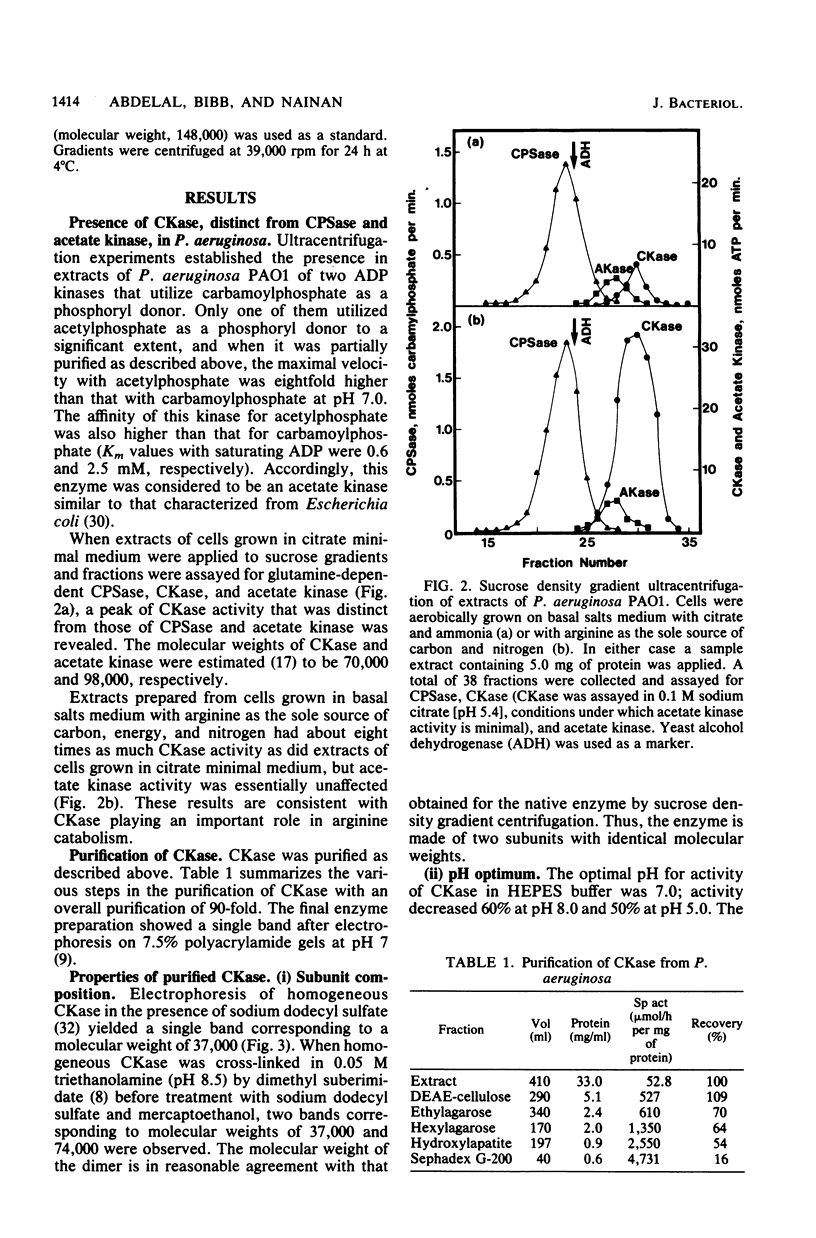
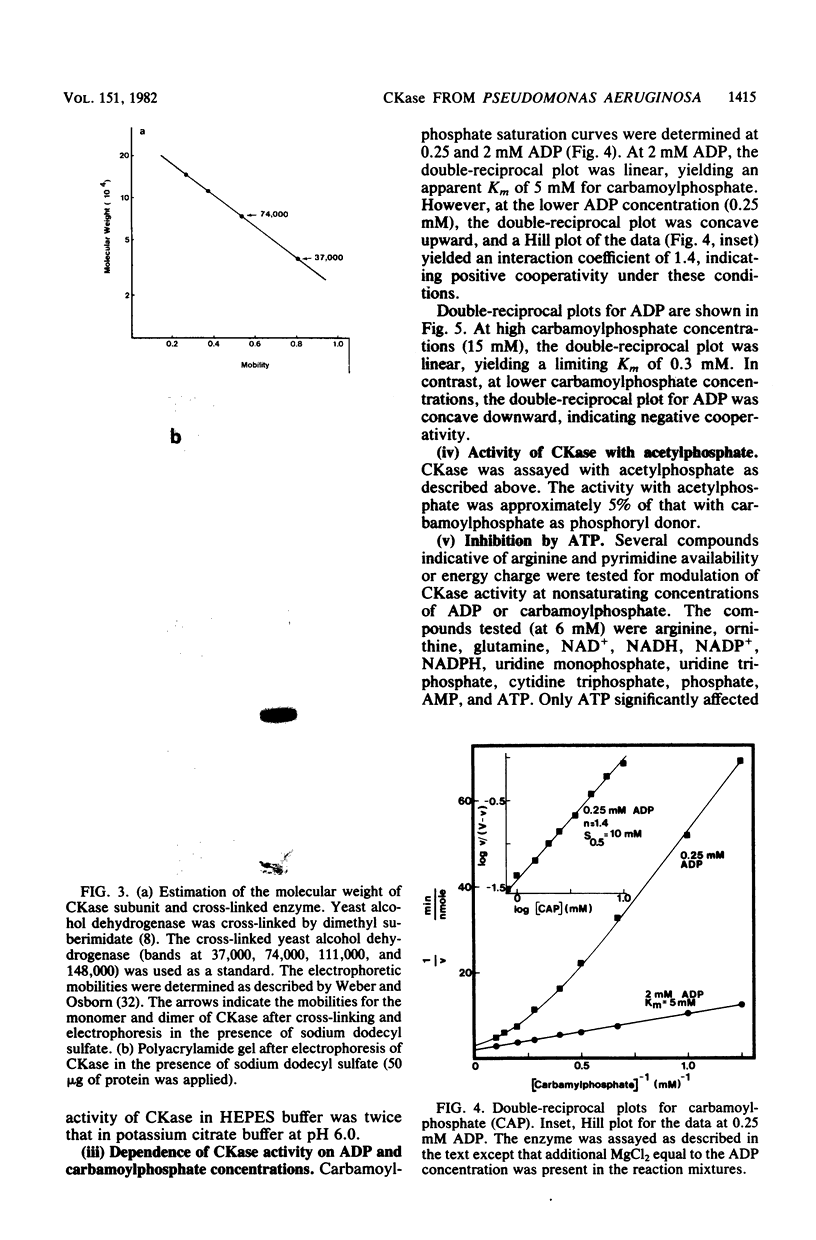
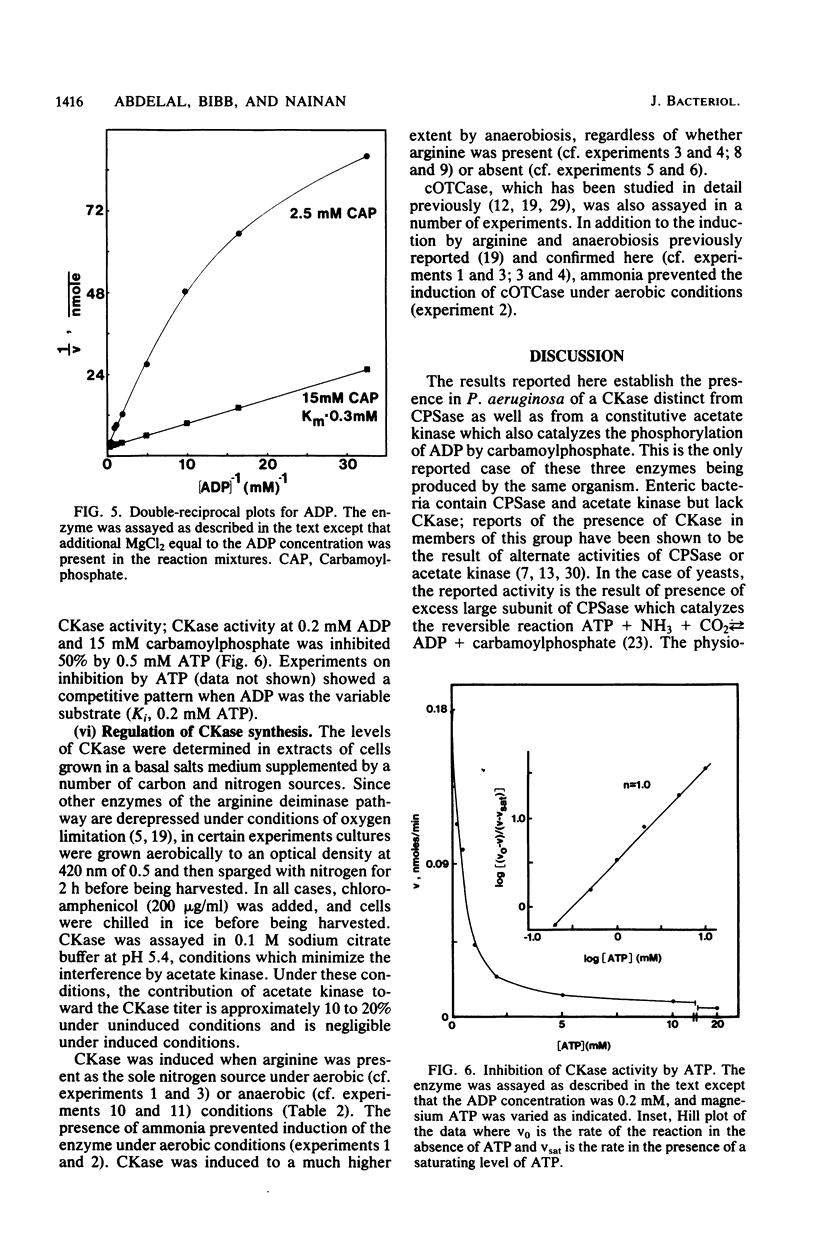
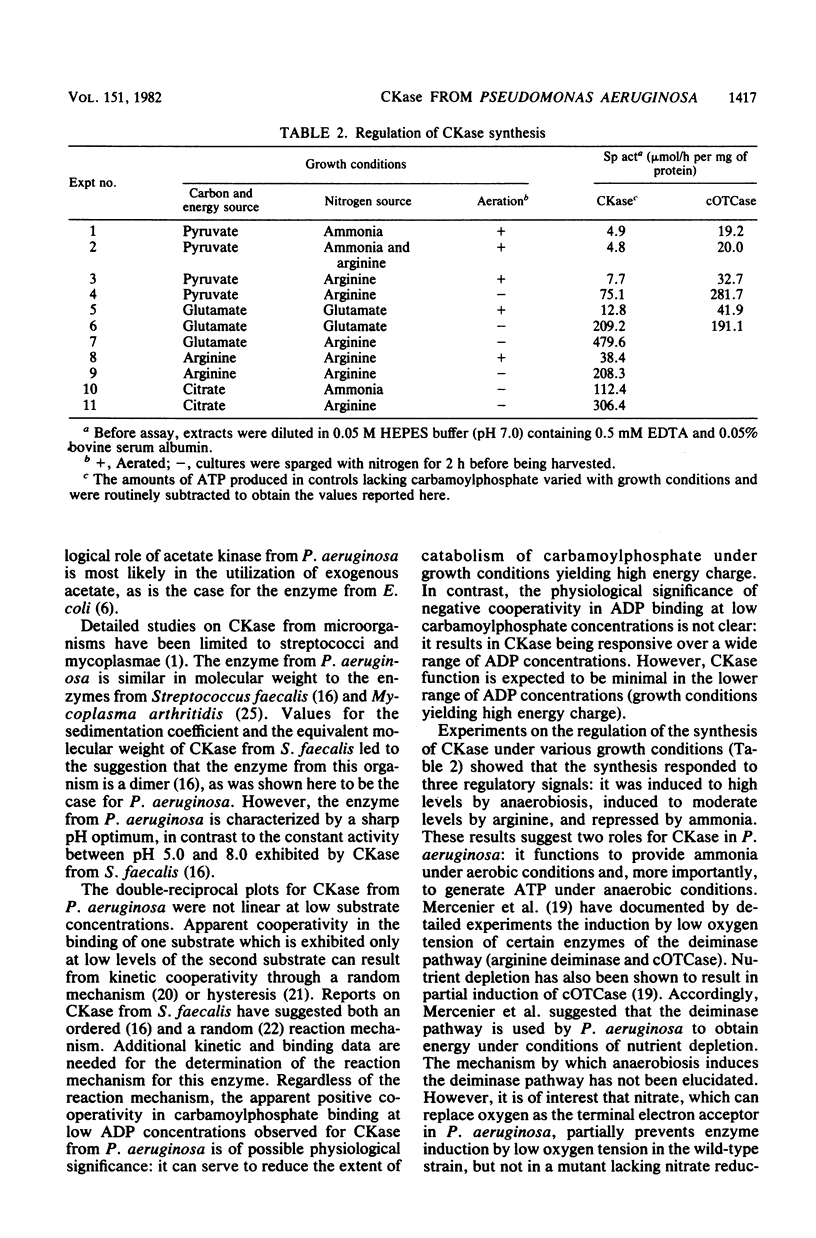
Pseudomonas aeruginosa PAO1 possessed a carbamate kinase (CKase) distinct from carbamoylphosphate synthetase as well as from a constitutive acetate kinase which also catalyzes the phosphorylation of ADP by carbamoylphosphate. CKase was purified to homogeneity. Polyacrylamide gel electrophoresis of cross-linked CKase in the presence of sodium dodecyl sulfate showed that the enzyme consists of two subunits with identical molecular weights (37,000). The optimal pH of enzyme activity is 7.0. The double-reciprocal plot for carbamoylphosphate was linear at 2 mM ADP, yielding an apparent Km of 5 mM. However, at 0.25 mM ADP, the plot was concave upward, and a Hill plot of the data yielded a coefficient of 1.4. This apparent cooperativity at low ADP concentrations might serve to reduce the extent of catabolism of carbamoylphosphate under growth conditions yielding high energy charge. Experiments on the regulation of synthesis under various growth conditions showed a response to three regulatory signals: CKase was induced to high levels by anaerobiosis, induced to moderate levels by arginine, and repressed by ammonia. Thus, CKase expression is regulated in a manner that allows the enzyme to function as a provider of ammonia under aerobic conditions and of ATP under anaerobic conditions. ATP was an effective inhibitor of CKase activity; this inhibition provides the cell with an effective mechanism for avoiding a futile cycle resulting from the simultaneous operation of CKase and carbamoylphosphate synthetase when cells are grown in the presence of exogenous arginine.
Full text
PDF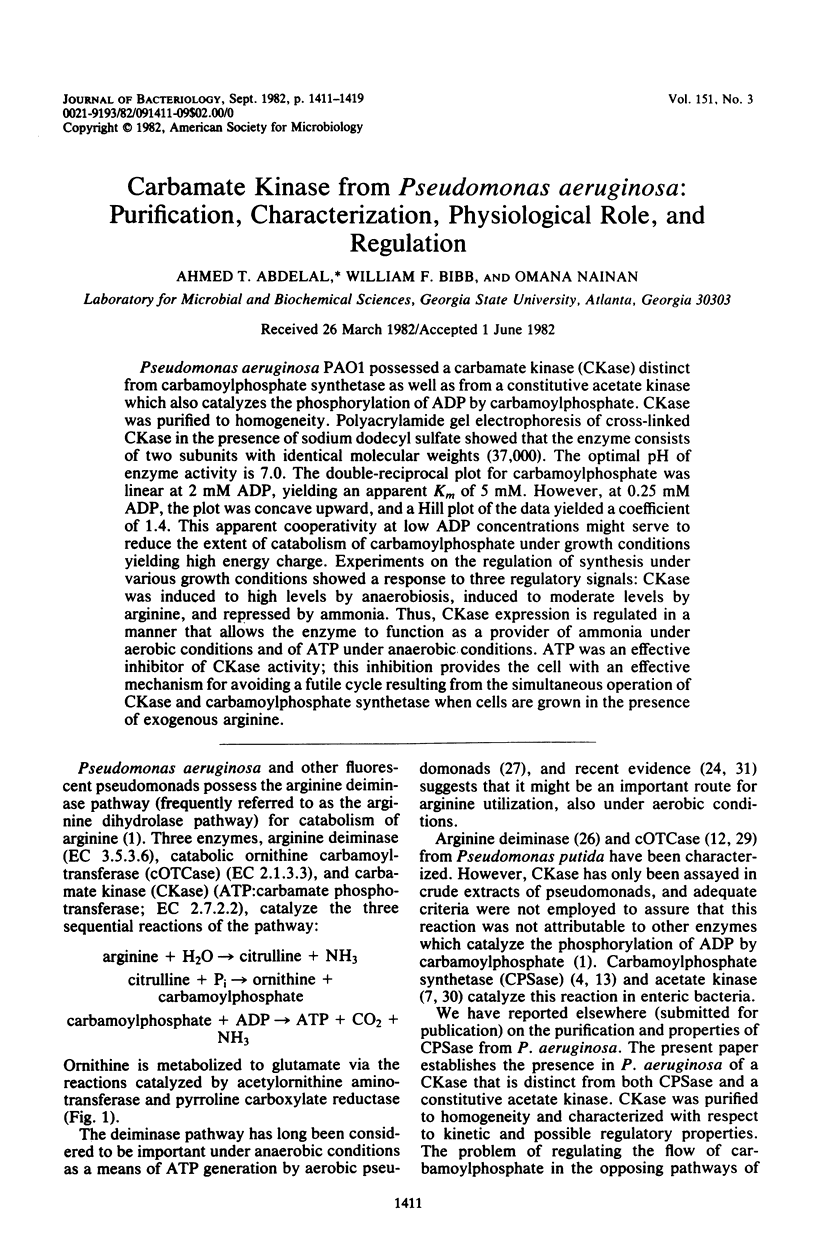



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelal A. T. Arginine catabolism by microorganisms. Annu Rev Microbiol. 1979;33:139–168. doi: 10.1146/annurev.mi.33.100179.001035. [DOI] [PubMed] [Google Scholar]
- Abdelal A. T., Ingraham J. L. Carbamylphosphate synthetase from Salmonella typhimurium. Regulations, subunit composition, and function of the subunits. J Biol Chem. 1975 Jun 25;250(12):4410–4417. [PubMed] [Google Scholar]
- Adair L. B., Jones M. E. Purification and characteristics of aspartate transcarbamylase from Pseudomonas fluorescens. J Biol Chem. 1972 Apr 25;247(8):2308–2315. [PubMed] [Google Scholar]
- Anderson P. M., Meister A. Bicarbonate-dependent cleavage of adenosine triphosphate and other reactions catalyzed by Escherichia coli carbamyl phosphate synthetase. Biochemistry. 1966 Oct;5(10):3157–3163. doi: 10.1021/bi00874a012. [DOI] [PubMed] [Google Scholar]
- Broman K., Lauwers N., Stalon V., Wiame J. M. Oxygen and nitrate in utilization by Bacillus licheniformis of the arginase and arginine deiminase routes of arginine catabolism and other factors affecting their syntheses. J Bacteriol. 1978 Sep;135(3):920–927. doi: 10.1128/jb.135.3.920-927.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. D., Jones-Mortimer M. C., Kornberg H. L. The enzymic interconversion of acetate and acetyl-coenzyme A in Escherichia coli. J Gen Microbiol. 1977 Oct;102(2):327–336. doi: 10.1099/00221287-102-2-327. [DOI] [PubMed] [Google Scholar]
- Crane C. J., Abdelal A. T. Regulation of carbamylphosphate synthesis in Serratia marcescens. J Bacteriol. 1980 Aug;143(2):588–593. doi: 10.1128/jb.143.2.588-593.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas D., Holloway B. W., Schamböck A., Leisinger T. The genetic organization of arginine biosynthesis in Pseudomonas aeruginosa. Mol Gen Genet. 1977 Jul 7;154(1):7–22. doi: 10.1007/BF00265571. [DOI] [PubMed] [Google Scholar]
- Halleux P., Legrain C., Stalon V., Piérard A., Wiame J. M. Regulation of the catabolic ornithine carbamoyltransferase of Pseudomonas fluorescens. A study of the quaternary structure. Eur J Biochem. 1972 Dec 4;31(2):386–393. doi: 10.1111/j.1432-1033.1972.tb02545.x. [DOI] [PubMed] [Google Scholar]
- Hass D., Evans R., Mercenier A., Simon J. P., Stalon V. Genetic and physiological characterization of Pseudomonas aeruginosa mutants affected in the catabolic ornithine carbamoyltransferase. J Bacteriol. 1979 Sep;139(3):713–720. doi: 10.1128/jb.139.3.713-720.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALMAN S. M., DUFFIELD P. H., BRZOZOWSKI T. IDENTITY IN ESCHERICHIA COLI OF CARBAMYL PHOSPHOKINASE AND AN ACTIVITY WHICH CATALYZES AMINO GROUP TRANSFER FROM GLUTAMINE TO ORNITHINE IN CITRULLINE SYNTHESIS. Biochem Biophys Res Commun. 1965 Feb 17;18:530–537. doi: 10.1016/0006-291x(65)90786-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lienhard G. E., Secemski I. I. P 1 ,P 5 -Di(adenosine-5')pentaphosphate, a potent multisubstrate inhibitor of adenylate kinase. J Biol Chem. 1973 Feb 10;248(3):1121–1123. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. A kinetic study of the mechanism of crystalline carbamate kinase. J Biol Chem. 1966 Sep 25;241(18):4197–4208. [PubMed] [Google Scholar]
- Mercenier A., Simon J. P., Haas D., Stalon V. Catabolism of L-arginine by Pseudomonas aeruginosa. J Gen Microbiol. 1980 Feb;116(2):381–389. doi: 10.1099/00221287-116-2-381. [DOI] [PubMed] [Google Scholar]
- Mercenier A., Simon J. P., Vander Wauven C., Haas D., Stalon V. Regulation of enzyme synthesis in the arginine deiminase pathway of Pseudomonas aeruginosa. J Bacteriol. 1980 Oct;144(1):159–163. doi: 10.1128/jb.144.1.159-163.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neet K. E., Ainslie G. R., Jr Hysteretic enzymes. Methods Enzymol. 1980;64:192–226. doi: 10.1016/s0076-6879(80)64010-5. [DOI] [PubMed] [Google Scholar]
- Pandey V. N., Pradhan D. S. Reverse and forward reactions of carbamyl phosphokinase from Streptococcus faecalis R. Participation of nucleotides and reaction mechanisms. Biochim Biophys Acta. 1981 Aug 13;660(2):284–292. doi: 10.1016/0005-2744(81)90172-8. [DOI] [PubMed] [Google Scholar]
- Price C. W., Holwell J. H., Abdelal A. T. Purification and properties of the arginine-specific carbamoyl-phosphate synthase from Saccharomyces cerevisiae. J Gen Microbiol. 1978 May;106(1):145–151. doi: 10.1099/00221287-106-1-145. [DOI] [PubMed] [Google Scholar]
- Rahman M., Laverack P. D., Clarke P. H. The catabolism of arginine by Pseudomonas aeruginosa. J Gen Microbiol. 1980 Feb;116(2):371–380. doi: 10.1099/00221287-116-2-371. [DOI] [PubMed] [Google Scholar]
- SHOESMITH J. H., SHERRIS J. C. Studies on the mechanism of arginine-activated motility in a Pseudomonas strain. J Gen Microbiol. 1960 Feb;22:10–24. doi: 10.1099/00221287-22-1-10. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Berlin C. M., Sweeney E. W., Carroll W. R. The generation of energy by the arginine dihydrolase pathway in Mycoplasma hominis 07. J Biol Chem. 1966 May 25;241(10):2228–2236. [PubMed] [Google Scholar]
- Shibatani T., Kakimoto T., Chibata I. Crystallization and properties of L-arginine deiminase of Pseudomonas putida. J Biol Chem. 1975 Jun 25;250(12):4580–4583. [PubMed] [Google Scholar]
- Stalon V., Ramos F., Piérard A., Wiame J. M. Regulation of the catabolic ornithine carbamoyltransferase of Pseudomonas fluorescens. A comparison with the anabolic transferase and with a mutationally modified catabolic transferase. Eur J Biochem. 1972 Aug 18;29(1):25–35. doi: 10.1111/j.1432-1033.1972.tb01953.x. [DOI] [PubMed] [Google Scholar]
- Stalon V., Ramos F., Piérard A., Wiame J. M. The occurrence of a catabolic and an anabolic ornithine carbamoyltransferase in Pseudomonas. Biochim Biophys Acta. 1967 May 16;139(1):91–97. doi: 10.1016/0005-2744(67)90115-5. [DOI] [PubMed] [Google Scholar]
- THORNE K. J., JONES M. E. CARBAMYL AND ACETYL PHOSPHOKINASE ACTIVITIES OF STREPTOCOCCUS FAECALIS AND ESCHERICHIA COLI. J Biol Chem. 1963 Sep;238:2992–2998. [PubMed] [Google Scholar]
- Voellym R., Leisinger T. Role of 4-aminobutyrate aminotransferase in the arginine metabolism of Pseudomonas aeruginosa. J Bacteriol. 1976 Dec;128(3):722–729. doi: 10.1128/jb.128.3.722-729.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



