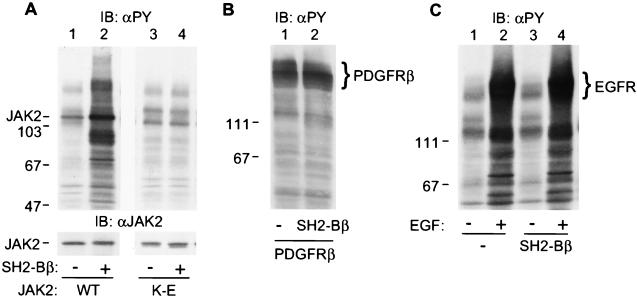
Figure 1.
SH2-Bβ stimulates tyrosyl phosphorylation of JAK2 and multiple other cellular proteins. (A) COS cells were cotransfected with plasmid (4 μg) encoding wild-type (lanes 1 and 2) or kinase-inactive JAK2 (lanes 3 and 4) and either control plasmid (10 μg) or plasmid (10 μg) encoding SH2-Bβ. After 48 hr, cells were lysed in lysis buffer. Proteins (40 μg) in the lysates were resolved by SDS/PAGE and immunoblotted with αPY (Upper) and reprobed with αJAK2 (Lower). (B) COS cells were cotransfected with plasmid (5 μg) encoding the β subunit of PDGF receptor with control plasmid (10 μg) or plasmid (10 μg) encoding SH2-Bβ. Proteins (40 μg) in the cell lysates were resolved by SDS/PAGE and immunoblotted with αPY. (C) COS cells were transfected with either control plasmid (10 μg) or plasmid (10 μg) encoding SH2-Bβ. Twenty-four hours after transfection, cells were deprived of serum overnight and stimulated with 100 ng/ml EGF for 10 min. Proteins (40 μg) in the lysates were resolved by SDS/PAGE and immunoblotted with αPY. The migration of molecular weight standards (×10−3) is indicated.