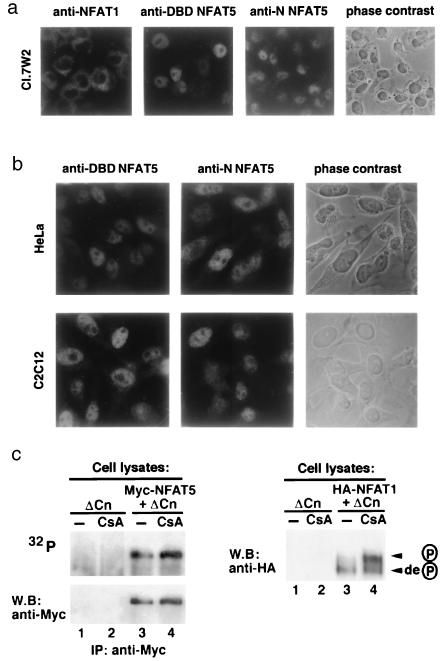
Figure 4.
Nuclear localization and phosphorylation status of NFAT5. (a) Nuclear localization of endogenous NFAT5 in a resting murine T cell clone. The first three panels show immunocytochemical staining of Cl.7W2 murine T cells with anti-NFAT1, or antisera against the N-terminal region (anti-N) or DNA-binding domain (anti-DBD) of NFAT5. (Right) Phase-contrast photomicrograph of the field stained with anti-N of NFAT5. (b) Nuclear localization of endogenous NFAT5 in resting HeLa (fibroblast, Upper) and C2C12 (myoblast, Lower) cells. (Left and Middle) Antisera against the NFAT5 DNA-binding domain (anti-DBD) or N-terminal region (anti-N) of NFAT5. (Right) Phase-contrast photomicrograph of the same field stained with anti-N. (c) Analysis of NFAT5 phosphorylation. (Upper) 293 cells expressing ΔCn or Myc-NFAT5-GFP and ΔCn were metabolically labeled with 32P-orthophosphate in the absence or presence of 1 μM CsA. NFAT5 was immunoprecipitated (IP) with anti-Myc and detected by autoradiography (32P) and Western blotting (W.B.). (Lower) In a parallel experiment, activity of ΔCn and inhibition by CsA were monitored by expressing hemagglutinin (HA)-tagged NFAT1 in 293 cells and evaluating its phosphorylation status in SDS-lysates by Western blotting with anti-HA. Arrows indicate the dephosphorylated and phosphorylated forms of NFAT1.