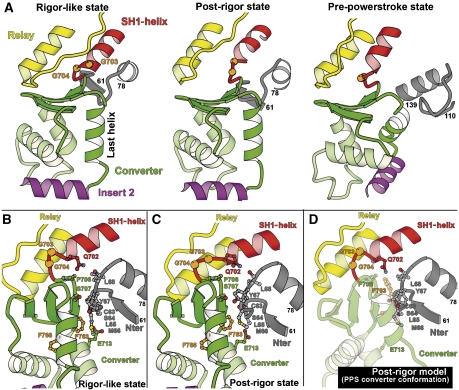
Figure 3.
The converter conformation of myosin VI. (A) The converter conformation of myosin VI is unchanged between the rigor-like state and the post-rigor state. Note also that the converter interacts with the same N-terminal subdomain (gray) residues in these two states. In contrast, a novel folding of the converter drastically changes the orientation of the helices in the pre-powerstroke state (Ménétrey et al, 2007). Also, a different region of the N-terminal subdomain interacts with the converter. This novel conformation results in a reorientation of insert 2 and thus of the myosin VI lever arm in this state. (B, C) In the rigor-like state (B) and in the post-rigor state (C), the converter makes similar interactions with the C63-L68 loop of the N-terminal subdomain. (D) Depicted is a model that demonstrates that the pre-powerstroke conformation of the converter is incompatible with the post-rigor structure, as it would create major steric clashes with the N-terminal subdomain region.