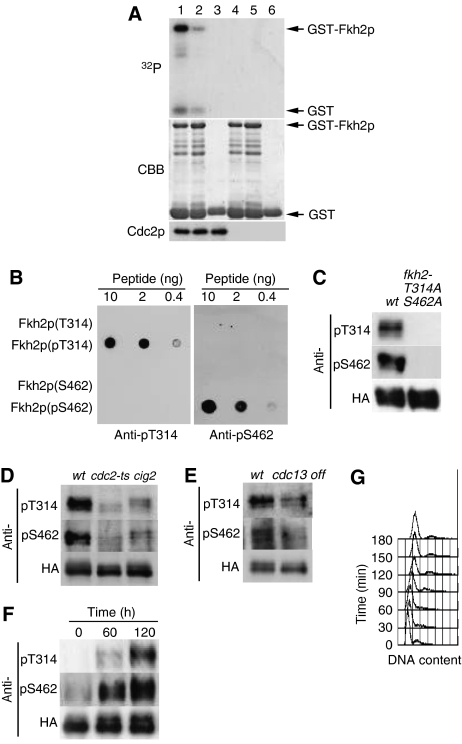
Figure 4.
Phosphorylation of Fkh2p on T314 and S462 by Cdc2p in vitro and in vivo. (A) Kinase assays were performed with Cdc2p precipitates prepared from protein extracts of exponentially growing cells expressing hemagglutinin epitope (HA)-tagged forms of Cdc2p (HM6118; lanes 1–3) or not expressing HA (HM6; lanes 4–6) with anti-HA antibody. Substrates (lanes 1–3, respectively) included GST–Fkh2p(305–492), GST–Fkh2p(305–492) containing T314A, S462A, and S481A mutations, or GST alone. Reaction mixtures were separated by SDS–polyacrylamide gel electrophoresis, and proteins were detected by staining with Coomassie brilliant blue (CBB) and autoradiography (32P). Arrows indicate GST and the GST–Fkh2p fusion proteins. The Cdc2p input into each reaction mixture was also examined separately by Western blotting. (B) Various amounts (10, 2, or 0.4 ng) of Fkh2p peptides containing phosphorylated or nonphosphorylated T314 or S462 were spotted onto a nitrocellulose membrane and subjected to immunodetection with affinity-purified antibodies (anti-pT314 and anti-pS462) generated in response to the corresponding phosphorylated peptides. (C) Cells expressing HA-tagged forms of wild-type Fkh2p (HM5145) or the Fkh2p(T314A,S462A) mutant (HM5722) were grown to mid-log phase at 30°C. Cell lysates were then subjected to immunoprecipitation with antibodies to HA, and the resulting precipitates were subjected to immunoblot analysis with anti-pT314, anti-pS462, and anti-HA, as indicated. (D) Cells expressing HA-tagged Fkh2p were either grown to mid-log phase in EMM2 at 24°C and then incubated at 36.5°C for 7 h (wt, HM5145; cdc2-ts, HM5444) or grown as in (C) (cig2, HM5530). Cell lysates were subjected to immunoprecipitation and immunoblot analysis as in (C). (E) Cells expressing HA-tagged Fkh2p (wt, HM5146; cdc13 off, HM5554) were grown to mid-log phase in EMM2 at 30°C, after which thiamine was added to the culture medium to switch off cdc13+ expression and the cells were incubated for an additional 5 h. Cell lysates were subjected to immunoprecipitation and immunoblot analysis as in (C). (F) Cells expressing HA-tagged Fkh2p (HM6107) were synchronized in G1 by transient temperature arrest and samples taken every 1 h upon release to the permissive temperature. Cell lysates were subjected to immunoprecipitation and immunoblot analysis as in (C). (G) DNA content of the cells in (F) was determined by flow cytometric analysis.