Figure 4.
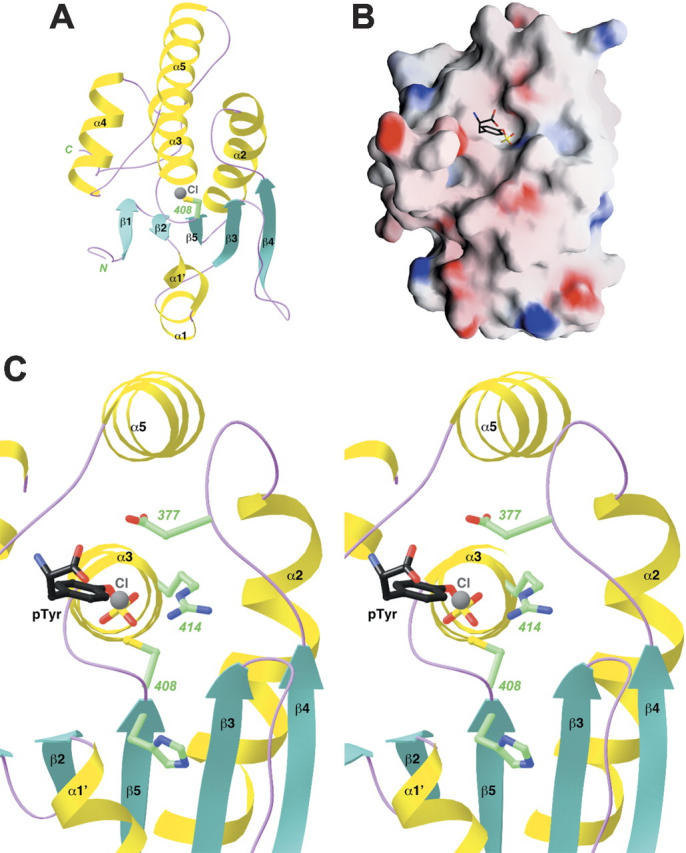
Structure of the catalytic domain of MKP5. (A) Schematic representation of the structure of the catalytic domain of MKP5. The catalytic Cys408 residue is shown in green. A chloride ion bound in the active site is shown in gray. (B) Molecular surface of the MKP5 catalytic domain. The binding mode of phosphotyrosine to PTP1B is shown (Puius et al. 1997). (C) A closeup of the active site region of the CD of MKP5. The side chains of residues His407, Cys408, Arg414, and Asp377 are shown in green. The chloride ion is shown in gray, and the phosphotyrosine (pTyr) observed in the structure of PTP1B is shown in black (Puius et al. 1997). Panels (A) and (C) were produced with Ribbons (Carson 1987) and panel (B) with Grasp (Nicholls et al. 1991).
