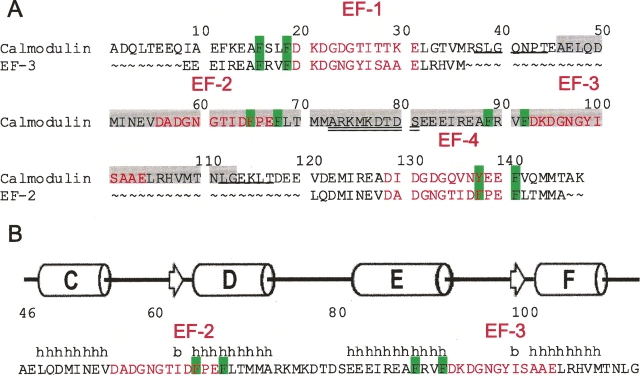
Figure 1.
The sequence and NMR-derived secondary structure of CaM2/3. (A) The amino acid sequence of CaM2/3 (residues 46–113) is highlighted in light gray within the sequence of full-length human CaM. The sequences of EF-3 and EF-2 are also aligned with those of EF-1 and EF-4, respectively, using the 12-residue Ca2+-binding segments (red type). Conserved aromatic residues important for forming part of the hydrophobic core of each domain are highlighted in green. Residues corresponding to the linker between the N- and C-domains of native CaM are double underlined. Residues corresponding to the loops between EF-hands in each domain are single underlined. (B) The amino acid sequence of CaM2/3 with the positions of (h) helices (cylinder) and (b) β-strands (arrows) identified by PROMOTIF (Hutchinson and Thornton 1996) within the ensemble of NMR-derived structures for CaM2/3.