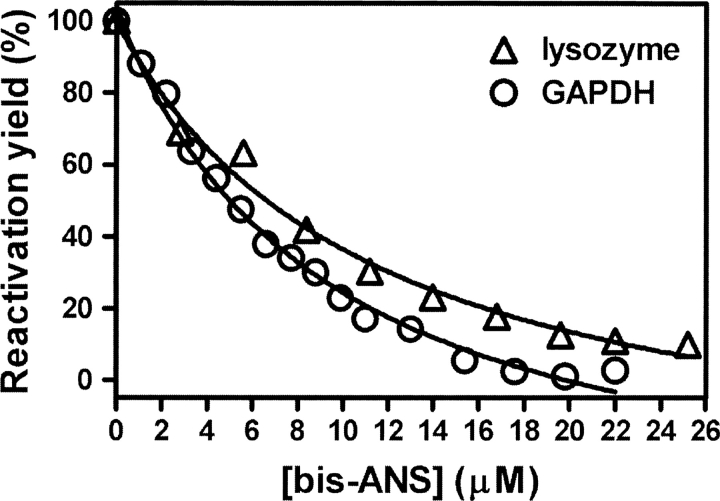
Figure 3.
Bis-ANS inhibits chaperone activity of TF. Refolding of GdnHCl-denatured GAPDH (○) or lysozyme (▵) was initiated by 50-fold dilution (for GAPDH) or 20-fold dilution (for lysozyme) into 0.1 M phosphate buffer (pH 7.5) containing 12 μM TF (for GAPDH) or 15 μM TF (for lysozyme) and 0–25 μM bis-ANS. The final concentrations of GAPDH and lysozyme in the refolding buffer were 2.8 and 10 μM, respectively. The GAPDH reactivation mixtures were first kept at 4°C for 30 min and then for a further 3 h at 25°C before samples were taken for assay of activity (Huang et al. 2000). The lysozyme reactivation mixtures were kept at 25°C for 5 h before samples were taken for assay of activity (Huang et al. 2002). The TF-assisted reactivation yield of GAPDH or lysozyme without bis-ANS was set as 100%.