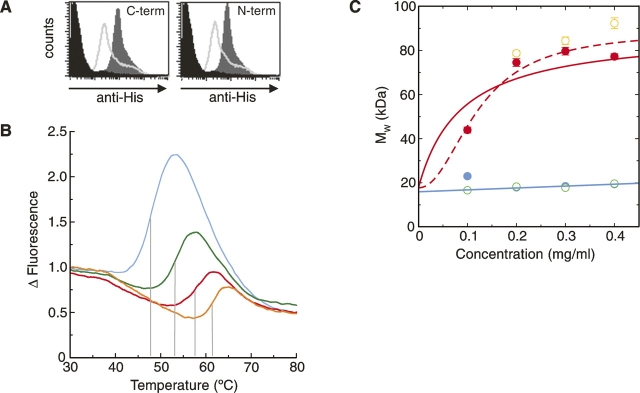
Figure 4.
Biophysical studies of refolded dectin-1. (A) FACS-based assay showing β-glucan binding by refolded dectin-1 constructs. The dark gray region corresponds to dectin-1 binding, the lighter gray line indicates inhibition by glucan phosphate, and the black region corresponds to the negative control. (B) Thermal shift profiles show dectin-1 binds β-glucan and divalent cations. Measurements of dectin-1 alone (blue), with Ca2+/Mg2+ (green), with laminarin (red), and with both Ca2+/Mg2+ and laminarin (orange) are shown. The shift in melting temperature, indicated by the gray lines, reflects the increased energy required to melt the protein in the presence of the various ligands. (C) AUC measurements demonstrate that dectin-1 forms multimeric complexes in the presence of laminarin. Measurements, colored as in B, were taken at 21,000 rpm and 280 nm. In the absence of laminarin (blue and green measurements), the molecular weights suggest monomeric species, whereas in the presence of laminarin (red and orange), there is an obvious increase in molecular weight. Two possible curve fits for the measurements taken with dectin-1 plus laminarin (in red) are shown (for details, see text).