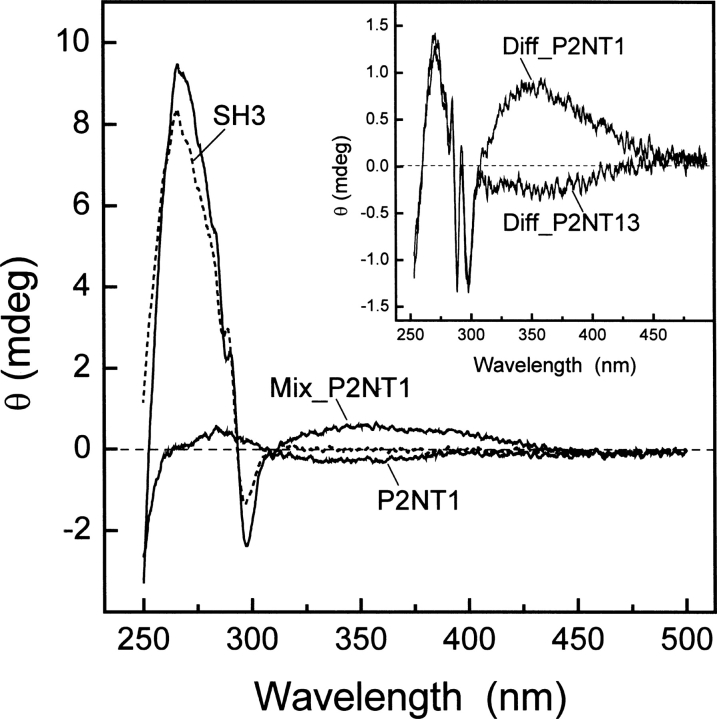
Figure 5.
Binding of NT analogs of P2 to Myo3-SH3 monitored by circular dichroism. CD spectra of free Myo3-SH3 (65 μM, ---) and P2NT1 (168 μM, —) were recorded in 5 mM Tris-HCl buffer (pH 8.0) containing 0.1% (w/v) PEG 8000, 0.2 M NaCl. Myo3-SH3 and P2NT1 were alternatively mixed in the same molar ratio (65 μM:168 μM) to yield ∼85% of bound SH3 (Mix_P2NT1, —). (Inset) Difference spectra (Diff_P2NT1 and Diff_P2NT13) obtained by subtracting the theoretical sum spectra of free SH3 and NT peptides from the corresponding spectra of the experimental complexes (Mix_P2NT1 and Mix_P2NT13). Measurements were carried out at 25° ± 0.2°C in a 0.5-cm quartz cuvette and subtracted for the corresponding baseline. Ellipticity data, θ, were expressed in millidegrees (mdeg), without further normalization.