Figure 1.
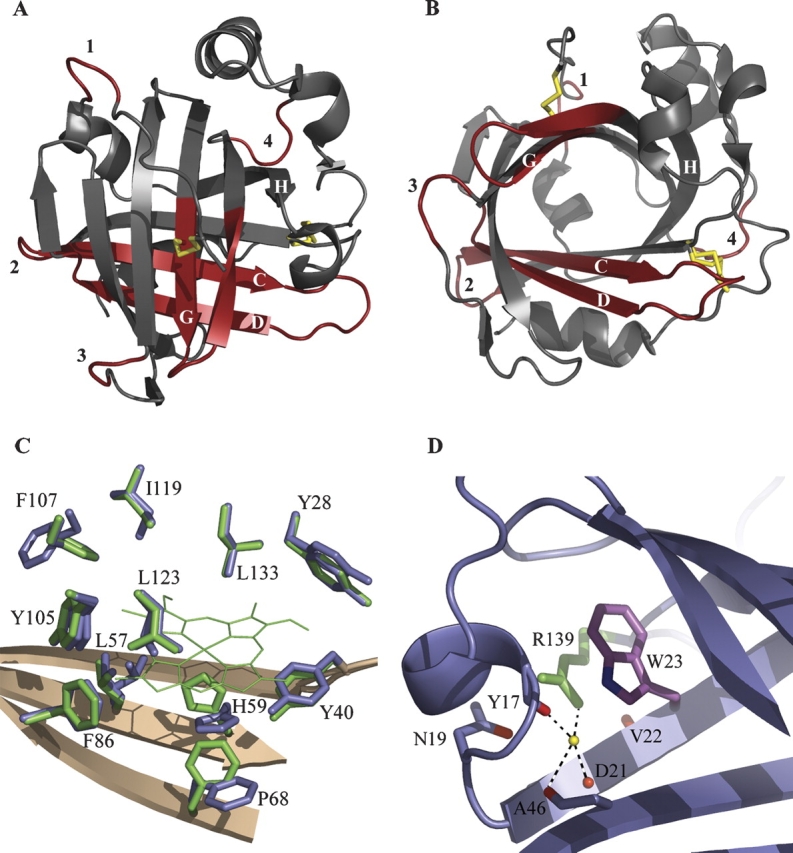
Crystal structure of apo-NP4. (A) Ribbon diagram highlighting changes. Subtle differences in the carbon-alpha backbone between apo- and holo-NP4 are highlighted in red. Labels 1–4 refer to the four loops displaying the largest changes (residues 9–12, 47–53, 100–103, and 140–143, respectively). Disulfide bonds are indicated with yellow bars. (B) As in A, emphasizing the open barrel. The disulfide bond between Cys 2 and Cys 122 (shown near the loop 1 label) occupies a single conformation, while that between Cys 41 and Cys 171 (shown near the loop 4 label) occupies two conformations. (C) Heme contacting residues in apo- and holo-NP4. Heme-pocket side chains of apo- and holo-NP4 are shown in blue and green, respectively. Heme in holo-NP4 is shown in green (thin lines) with heme propionates omitted for clarity. (D) Tryptophan–arginine interaction in NP4 (typical for the lipocalin fold). Tryptophan 23 and arginine 139 are in purple and green, respectively. Carbonyl oxygens in direct or water-mediated interaction (yellow sphere) with arginine 139 are in red.
