Figure 2.
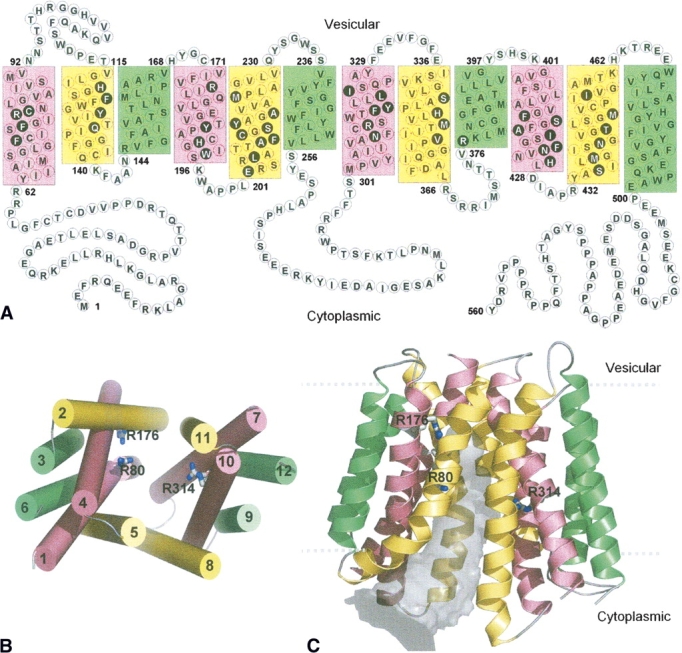
(A) Proposed topology of VGLUT1 based on transmembrane segment prediction and topology of bacterial MFS proteins. Residues in filled black circles face the center of the pore that separates the N- and C-terminal domains in 3D. The 12 transmembrane helices in this and the following figures are colored according to the grouping of helices in the oxalate transporter (Hirai et al. 2002), based on their symmetrical positions in the structure. Green: straight-spanning peripheral helices that are not involved in defining the pore. Yellow and pink: curved helices, at the interface of the two domains, lining the pore. (B) Packing of the helices viewed from the cytoplasmic side. The 12 helices are numbered sequentially. (C) Cartoon diagram of the human VGLUT1 model. The 12 transmembrane helices form pseudo-symmetrical domains, separated by a pore (gray volume) that is open to the cytoplasmic side. Three arginine residues (R80 from helix 1, R176 from helix 4, and R314 from helix 7) that are exposed to the pore are shown in sticks. The highly variable first and last 60 residues of the N- and C-terminal are not shown.
