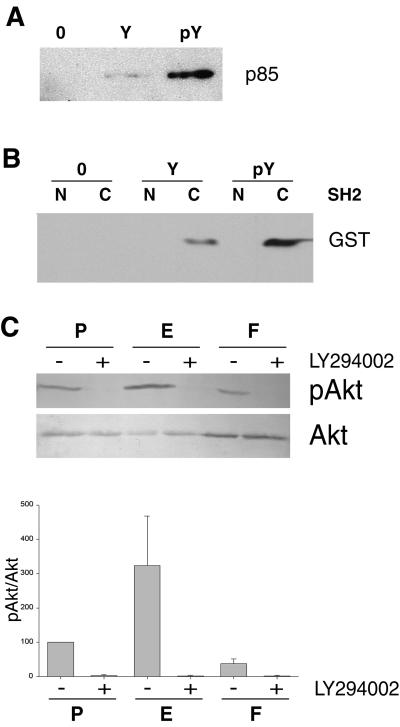
Figure 3.
Activation of the PI 3-kinase/Akt pathway requires ezrin phosphorylation at residue Tyr-353. (A) Binding of p85 to a phosphorylated peptide corresponding to amino acids 348–358 of ezrin. A lysate from LLC-PK1 cells was incubated with either the beads alone (0), the immobilized peptide with the nonphosphorylated residue Tyr-353 (Y), or phosphorylated residue Tyr-353 (pY). Bound material was analyzed by Western blotting with polyclonal anti-p85 antibodies. (B) Binding of the carboxyl-terminal SH2 domain of p85 to the peptide containing the phosphorylated Tyr-353. The p85 amino-terminal SH2 (N) and the carboxyl-terminal SH2 (C) domains fused to GST were incubated with either the beads alone (O), the peptide with the nonphosphorylated residue Tyr-353 (Y), or the peptide with the phosphorylated residue Tyr-353 (pY). Bound material was analyzed by Western blotting with an anti-GST serum. (C) Analysis of Akt activity in three-dimensional cultures. Serum-starved LLC-PK1 clones were embedded in collagen gels. Lysates from P, E, and F cells treated with either LY294002 (+) or DMSO (−) were analyzed with either anti-phosphospecific Akt (Ser-473) (pAkt) or anti-Akt (Akt) polyclonal antibodies. Signals were analyzed by densitometry. The pAkt/Akt ratio was calculated and set to 100% for untreated P cells. Mean ± SEM of three independent experiments are presented in the histogram.