Figure 1.
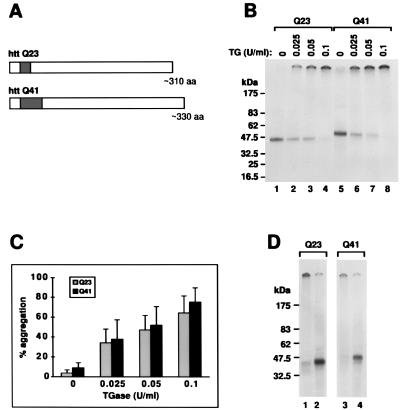
httQ23 and httQ41are soluble and form aggregates in the presence of TGase. (A) Constructs used in in vitro translation. Each construct begins with the N-terminal methionine of the htt protein. The dark bar indicates the location and size of the polyglutamine repeats. (B) In vitro expression of httQ23 and httQ41. RNA synthesized from the T7 promoter of pCDNA3 containing the httQ23 or httQ41 constructs was translated in the presence of [35S]methionine in a rabbit reticulocyte lysate system, producing soluble products of the expected molecular weights (lanes 1 and 5, respectively). Incubation in the presence of increasing concentrations of TGase produced increasing amounts of an aggregate that remained at the top of the gel (lanes 2–4 for httQ23 and lanes 6–8 for httQ41). (C) Quantitation of htt aggregation in the presence of TGase. Densitometry was performed on three similar gels as in B. The percentage of products that remained at the top of the gel within each lane is shown as a function of TGase concentration. The percentage is a mean of three separate experiments ± 1 SEM. There is no significant difference in the aggregation percentages between the two constructs. (D) Inhibition of TGase-mediated aggregation with an antibody against TGase. Lanes 1 and 2 contain in vitro translated httQ23 incubated in the presence of 0.1 unit/ml TGase. Lanes 3 and 4 contain in vitro translated httQ41 incubated in the presence of 0.1 unit/ml TGase. In addition, for lanes 2 and 4 during incubation with TGase, a monoclonal antibody that inhibits the activity of TGase was present at a concentration of 80 μg/ml.