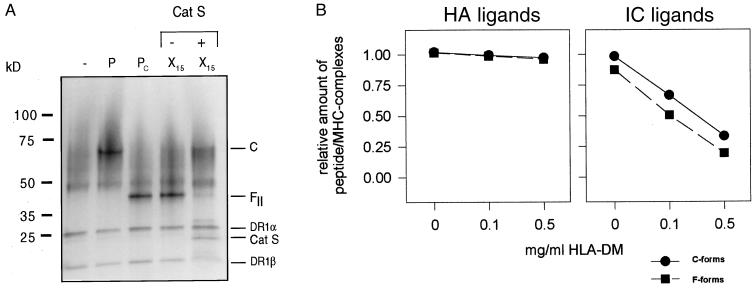
Figure 6.
Effect of cathepsin S and HLA-DM on C- and on F-forms of HLA-DR1/ligand complexes. HLA-DR1/ligand complexes were incubated with cathepsin S or soluble HLA-DM. (A) Conversion of an F-complex into the C-form by cathepsin S digest. FII-complexes, preformed by loading the HA-X15 ligand onto the HLA-DR1 molecules, were subjected to cathepsin S digest to remove the random peptide overhang. The gel shows the SDS/PAGE analysis of HLA-DR1/HA-X15 ligand complexes (lanes X15) before (−) and after (+) the treatment with cathepsin S. Also shown are the complexes formed with the HA306-318 peptide (lane P) and the HA306-338 peptide (PC). (B) HLA-DM mediated release of ligands from C- or F-ligand complexes. F- (closed square) or C-HLA-DR1/ligand complexes (closed circle) were formed with biotinylated ligands containing the HA306-318 (Left) or the IC106-120 binding epitope (Right). HA306-318 and IC106-120 were used for the formation of C-complexes, and the F complexes were generated with the HA teg-dimer or the IC97-120 peptide. The ligand complexes were incubated with the indicated amounts of soluble HLA-DM, and the release of ligands was measured by ELISA by determining the amount of stable MHC ligand complexes left after the incubation.