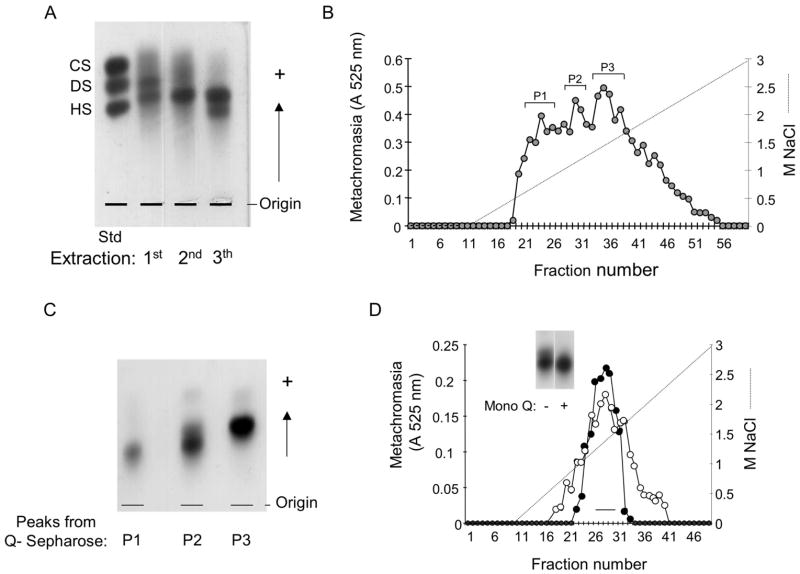
Figure 1. Purification of the S.plicata heparin.
A. Agarose gel electrophoresis of the crude glycans isolated from the body of S.plicata. The body of the ascidian was submitted to 3 proteolytic digestions and the glycans (~1.5 μg, as uronic acid) obtained in each extraction were applied to a 0.5% agarose gel in 0.05 M 1,3-diaminopropane/acetate (pH 9.0), along with a mixture of standard glycosaminoglycans, containing chondroitin sulfate (CS), dermatan sulfate (DS) and heparan sulfate (HS). B. Ion-exchange chromatography on Q-sepharose. The glycans from the 3rd extraction (~8 mg) were fractionated on a Q-sepharose column, as described in Material and Methods. Fractions eluted with different NaCl concentrations were pooled, and denominated P1, P2 and P3. C. Agarose gel electrophoresis of the fractions from the Q-sepharose column. The glycans pooled in P1, P2 and P3 (~1.5 μg, as uronic acid) were applied to a 0.5% agarose gel in 0.05 M 1,3-diaminopropane/acetate (pH 9.0) and submitted to an electophoretic run as described in Material and Methods. D. Ion-exchange chromatography on a Mono Q column. About 2 mg of P2 from the Q-sepharose column of mammalian (-●-) or S.plicata heparin (-○-) were applied to a Mono Q column and the chromatography developed as described in Material and Methods. The fractions eluted at ~ 1.2 M NaCl were pooled as indicated by the horizontal bar and analized by agarose gel electrophoresis (insert), as described earlier.