Figure 7.
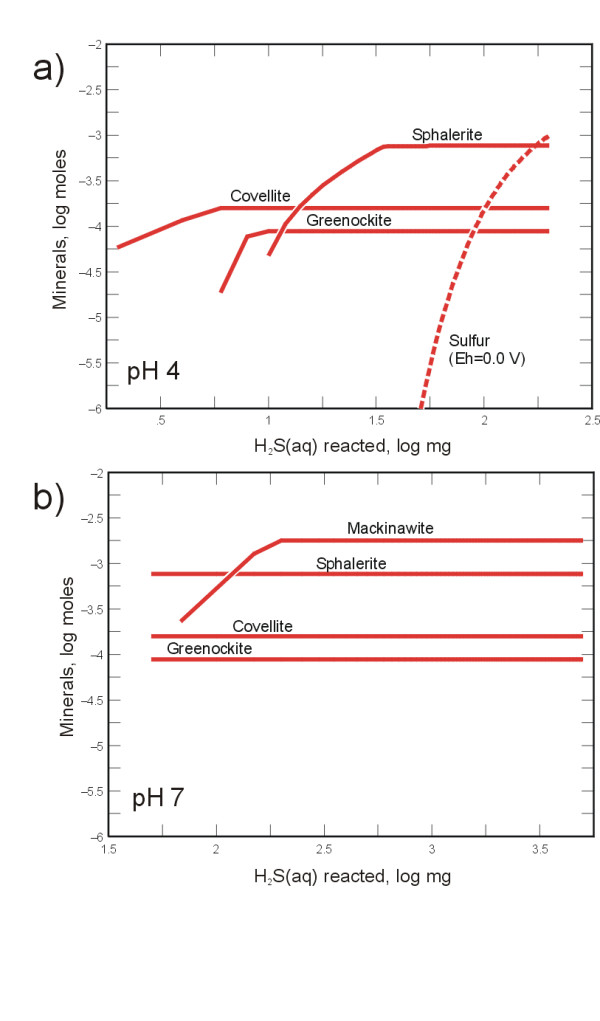
Reaction path modeling results showing trends in mineral precipitation as H2S(aq) is added to solutions containing Cd, Cu, and Zn. a) At pH 4, covellite (CuS), greenockite (CdS), and sphalerite (ZnS) precipitate, but mackinawite (FeS) remains undersaturated. b) At pH 7, all metal sulfides precipitate. The model was also run with the Eh fixed at 0.0 mV. At this condition elemental sulfur (dashed line) precipitation is favored at pH 4 following metal sulfide precipitation. Initial metals concentrations were Fe (150 mg L-1), Zn (100 mg L-1), Cd and Cu (15 mg L-1).
