Figure 2.
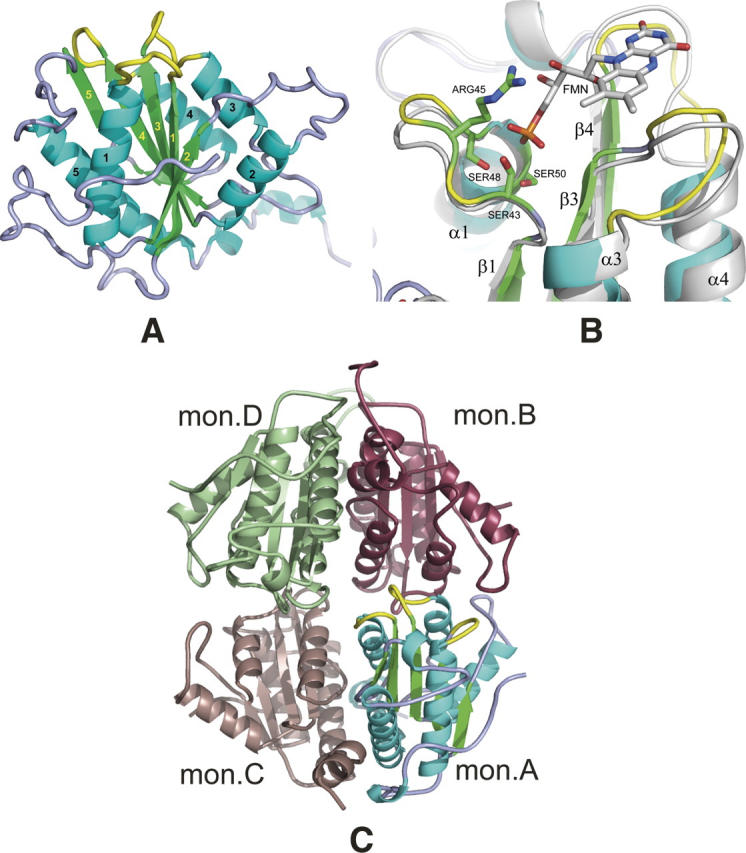
Structural overview. (A) General view of the S. flexneri ArsH protein. Secondary structures are colored in cyan (helixes), green (strands), and light blue/yellow (loops). Loops forming a binding site are shown in yellow. Labeling for β-strands and α-helixes is shown in yellow and black, respectively. (B) “Magic Fit” from Swiss-PDBViewer of the ArsH protein (colored) from S. flexneri with 1X77 FMN binding holoprotein (gray with colored FMN cofactor). Side chains of ArsH serine residues 43, 48, and 50 and arginine 45 are considered to participate in direct interaction with FMN phosphate group. Yellow-colored loops are also interacting with FMN and, probably, NADPH second cofactor. (C) General view of S. flexneri ArsH tetramer in the crystal, which is formed by two dimers A and C and B and D. Monomer A is colored as on the panel A, color codes for monomers B, C, and D are raspberry, dark salmon, and pale green, respectively.
