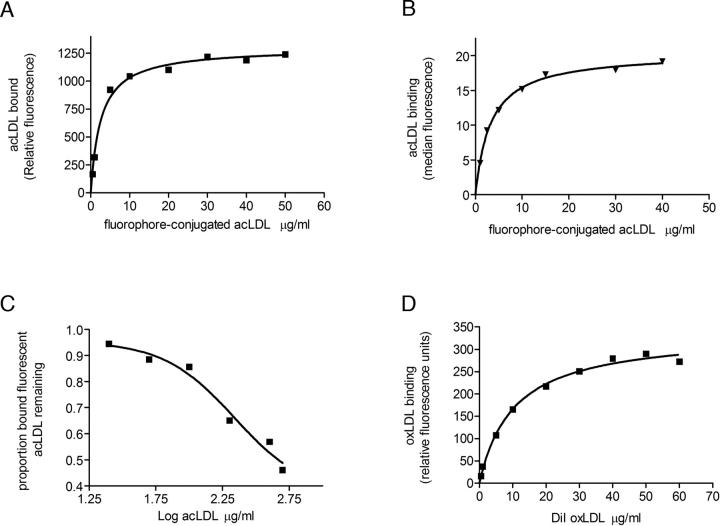
Figure 6.
Interaction of Cd36–12His with modified low-density lipoproteins. (A) In vitro binding of increasing concentrations of Alexa Fluor 488 or Bodipy acLDL to purified Cd36 in OG detergent micelles as described in Materials and Methods. A representative binding curve is depicted, with relative fluorescence units for ligand binding plotted as a function of ligand concentration. Curve fitting was conducted as described in Materials and Methods from which values for Kd were determined. Fluorophore-conjugated acLDL bound with a mean Kd ± s.e.m. = 2.98 ± 0.82 μg/mL (n = 7). (B) In vivo flow-cytometric analysis of the interaction of Cd36–12His, expressed transiently in HEK 293T cells, with Bodipy-acLDL over a range of concentrations. Median fluorescence of ligand bound to cells expressing Cd36–12His was plotted as a function of ligand concentration as described in Materials and Methods. A value for Kd of 5 μg/mL was obtained. (C) Displacement of fluorophore-conjugated acLDL (5 μg/mL) binding to purified Cd36 in OG detergent micelles by increasing concentrations of unlabelled acLDL. Representative data are presented as the proportion of bound fluorophore-conjugated acLDL remaining in the presence of increasing concentrations of unlabelled acLDL. Fluorophore-conjugated ligand was displaced by unlabelled acLDL with a mean EC50 = 150 ± 66 μg/mL (n = 4). (D) Saturation binding of DiI-oxLDL to purified Cd36:OG micelles. A representative binding curve is shown, and curve fitting was conducted as described in Materials and Methods to obtain a mean Kd ± s.e.m. = 10.44 ± 0.08 μg/mL (n = 2).