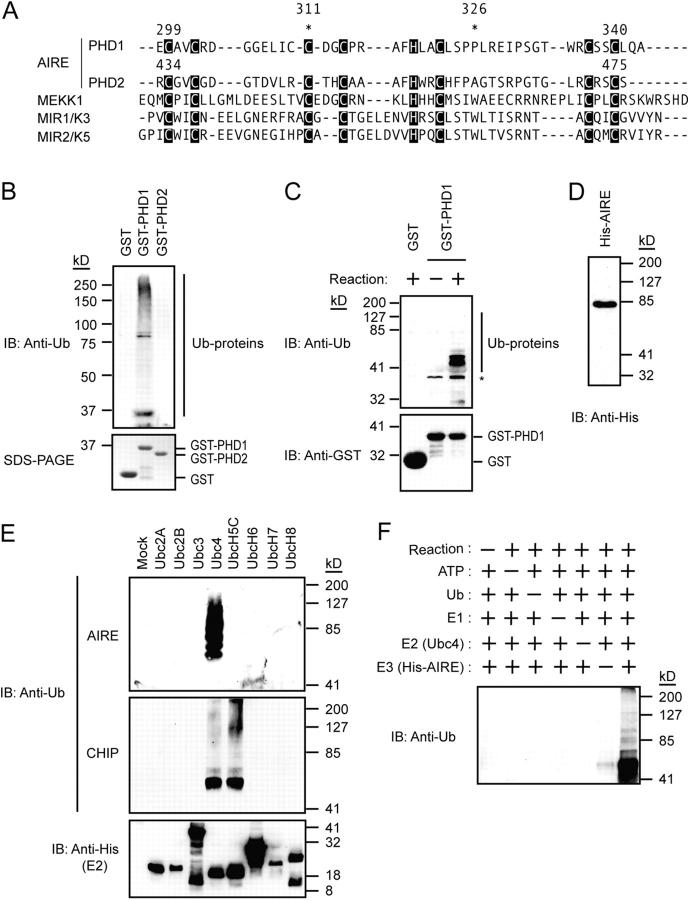
Figure 1.
AIRE mediates E3 ligase activity through PHD1. (A) The PHD1 and PHD2 domains of AIRE showed homology with the PHDs from other proteins showing E3 ligase activity. *, the position of disease-causing mutations (C311Y and P326Q) used in the study. (B) The GST-PHD1, but not GST-PHD2, fusion protein exhibited polyubiquitylated proteins when rabbit reticulocyte lysate was used for in vitro ubiquitylation assays (top). SDS-PAGE of GST-fusion proteins used in the assay (10% input) is shown on the bottom. (C) Recombinant E1 and E2 (Ubc4) were used for in vitro ubiquitylation assays (top). The ubiquitylation reactions were subjected to immunoblot analysis with antibodies against GST (bottom). E3 ligase activity mediated through PHD1 is incubation dependent. *, nonspecific bands. (D) The full-sized recombinant AIRE protein was successfully and homogeneously expressed in Sf21 cells using a baculovirus expression system. The purified recombinant AIRE protein was subjected to immunoblot analysis with an antibody against His6-tag. (E) Ubc4 E2 preference by the full-sized recombinant AIRE in in vitro ubiquitylation assays (top). CHIP, a U-box–type E3 ligase, is included to verify the specificity and biological activities of E2s (middle; reference 22). Equal amount of E2s were used in the reaction (bottom). (F) Specificity of E3 ligase activity mediated by the full-sized recombinant AIRE in in vitro ubiquitylation assays.