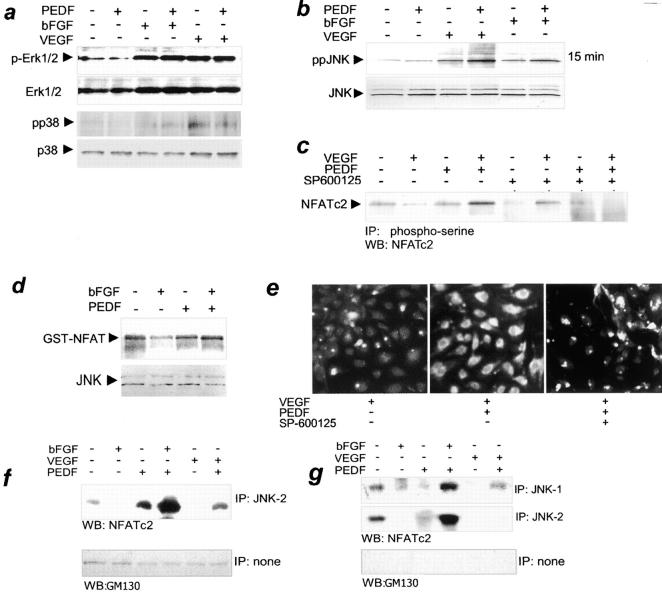
Figure 2.
The role of JNK kinases in NFATc2 deactivation by PEDF. (a) PEDF failed to activate p38MAPK or Erk1/2 kinases. Confluent HUVECs were stimulated with 200 pg/ml VEGF or 5 ng/ml bFGF. 10 nM PEDF was added where indicated. Cell extracts were analyzed by Western blotting with antibodies for active phosphorylated forms of Erk1/2 (p-Erk) and for dually phosphorylated p38 (pp38). The blots were reprobed for total Erk and p38 to ensure equal loading. The representative result of three independent experiments is shown. (b) PEDF enhanced JNK activation in stimulated ECs. HUVECs were treated as indicated with VEGF or bFGF + PEDF for 15 min. Cell extracts were resolved by SDS-PAGE and analyzed by Western blotting with antibodies for active, dually phosphorylated JNK (top, ppJNK) or total JNK (bottom) as a loading control. Three independent experiments were performed with similar results. (c) PEDF-dependent NFATc2 phosphorylation required JNK kinases. VEGF-stimulated HUVECs were treated with PEDF alone or in combination with 100 nM of SP600125, a generic JNK inhibitor. Cell lysates were immunoprecipitated with phosphoserine antibody and analyzed by Western blotting with NFATc2 antibody. Note the increase in NFATc2 phosphorylation by PEDF in stimulated ECs and its attenuation in the presence of JNK inhibitor. (d) Increased JNK activity in PEDF-treated cells. HUVECs were treated with bFGF (15 min, 5 ng/ml) and/or PEDF, and JNK activity was assessed by immunocomplex kinase assay with exogenous recombinant GST-NFATc2 substrate. Note the decrease in JNK activity in bFGF-treated ECs and the capacity for NFATc2 phosphorylation in cells treated with bFGF + PEDF. Western blot with JNK antibodies was performed to ensure equal loading. The result is representative of three independent experiments. (e) NFATc2 redistribution by PEDF was JNK dependent. HUVECs were plated on gelatinized coverslips; treated with indicated combinations of VEGF, PEDF, and JNK inhibitor SP600125; and stained for NFATc2. JNK blockade caused persistent NFATc2 nuclear localization (active state) despite PEDF treatment. (f and g) PEDF caused physical interaction between JNK and NFATc2. Quiescent or activated HUVECs (induced with VEGF or bFGF, as indicated) were treated for 15 min with PEDF. Nuclear or cytosolic extracts were precipitated with JNK-1– or JNK-2–specific antibody and analyzed by Western blotting with NFATc2 pAb. Purity of the fractions was determined by blotting with antibodies against GM130 protein. (f) PEDF increased interaction between JNK-2 and NFATc2 in the cytosol of stimulated ECs. (g) NFATc2 interacted with both JNK-1 and JNK-2 in the nuclei; note the increased complex formation by PEDF in stimulated but not in quiescent ECs.