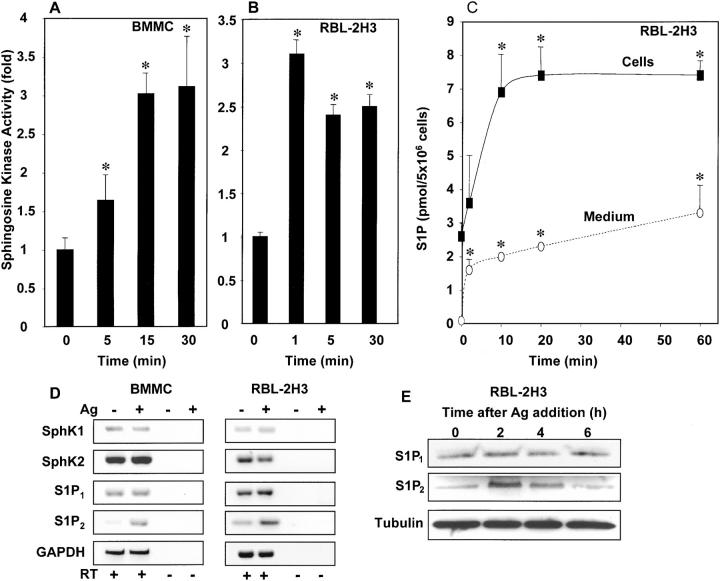
Figure 1.
FcɛRI cross-linking stimulates SphK and increases S1P. BMMCs (A) and RBL-2H3 cells (B and C) were sensitized with anti-DNP IgE and treated with DNP-HSA as described in Materials and Methods. At the indicated times, cells were lysed, and SphK activity was measured in the presence of 0.25% Triton X-100. Data are presented as fold increase ± SD. SphK activity in unstimulated BMMCs and RBL-2H3 cells were 1.5 ± 0.4 and 3.4 ± 0.4 pmol/min/mg, respectively. *, P < 0.05 by Student's t test. (C) Mast cells secrete S1P. RBL-2H3 cells were sensitized with anti-DNP IgE, washed, and treated with DNP-HSA in serum-free medium containing 4 mg/ml BSA. Mass levels of S1P in RBL-2H3 cells (closed symbols) and media (open symbols) were measured at the indicated times. *, P < 0.05 by Student's t test. (D and E) Expression of SphKs and S1PRs in mast cells. (D) RT-PCR analysis of expression of SphKs and S1PRs. Anti-DNP IgE-sensitized BMMCs and RBL-2H3 cells were stimulated without or with DNP-HSA (Ag). After 1 h at 37°C, RNA was extracted and SphKs, S1PRs, and glyceraldehyde-3–phosphate dehydrogenase (GAPDH) mRNAs were determined by RT-PCR. No PCR products were detected in the absence of RT. (E) IgE-sensitized RBL-2H3 cells were treated with Ag for the indicated times, and cell lysates were examined by Western blotting with anti-S1P2 or anti-S1P1 (Exalpha) and subsequently with antitubulin antibody to show equal loading as described in Supplemental Materials and Methods.