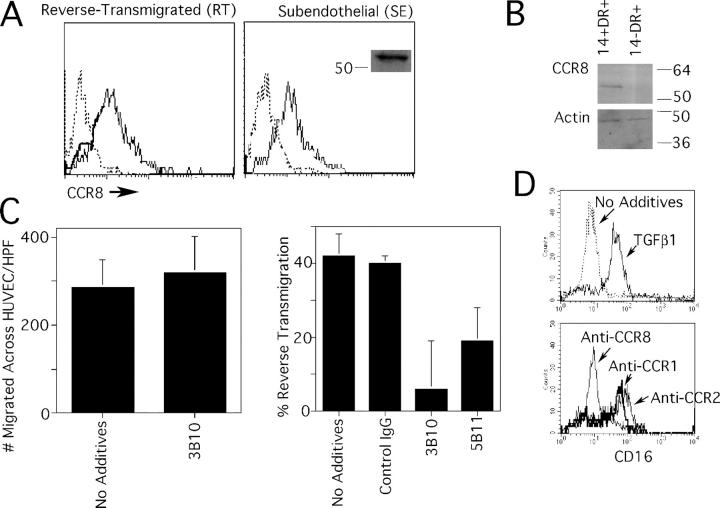
Figure 6.
Expression of CCR8 by human monocyte-derived cells and skin DCs, and its role in reverse transmigration. Monocytes were cocultured with endothelial cells grown on a type I collagen gel for 48 h, permitting the separation of the population into reverse-transmigrating DCs (RT) or subendothelial macrophages (SE). (A) Anti-CCR8 mAb 3B10 (thin solid line) reacted with both RT and SE monocyte-derived cells. Isotype-matched mAb was used to establish the baseline for negative staining (dotted line). Anti-CCR8 mAb 3B10 specifically recognized an ∼54 kD band by immunoblot, shown only for SE monocyte-derived cells (inset). (B) CD14+ DC precursors from human skin, but not CD14− DCs, were positive for CCR8 by immunoblot. Blotting for β-actin was conducted as a loading control. (C) Inclusion of neutralizing anti-CCR8 mAb 3B10 during the assay when monocytes traverse endothelium in the apical-to-basal direction had no effect (left), but 3B10 anti-CCR8 mAb and anti-CCR8 mAb 5B11 significantly (P < 0.005) inhibited reverse transmigration in more than five independent experiments. (D) CD16− monocytes were selected by magnetic depletion to remove CD16+ monocytes and cultured overnight in the presence or absence of TGFβ1 to induce CD16 (top). In some samples, 20 ng/ml TGFβ1 was added together with neutralizing mAbs to CCR1, CCR2, or CCR8. Expression of CD16 was monitored by flow cytometry. This result is representative of five experiments.