Figure 1.
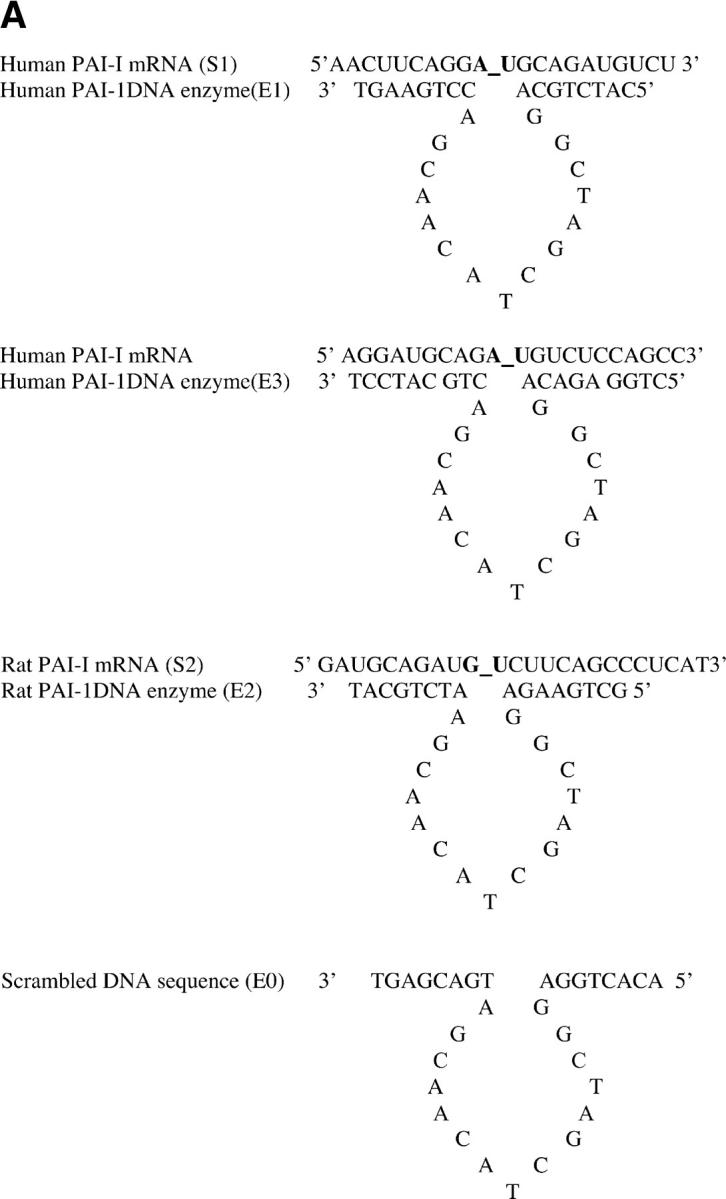
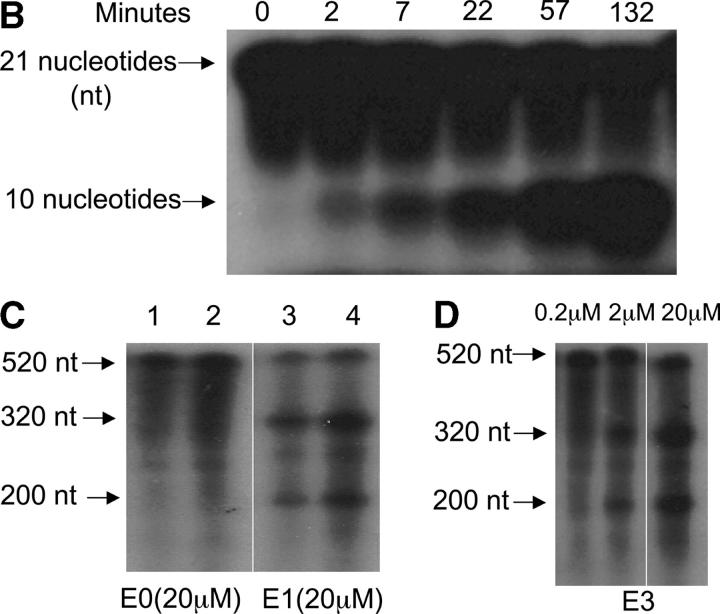
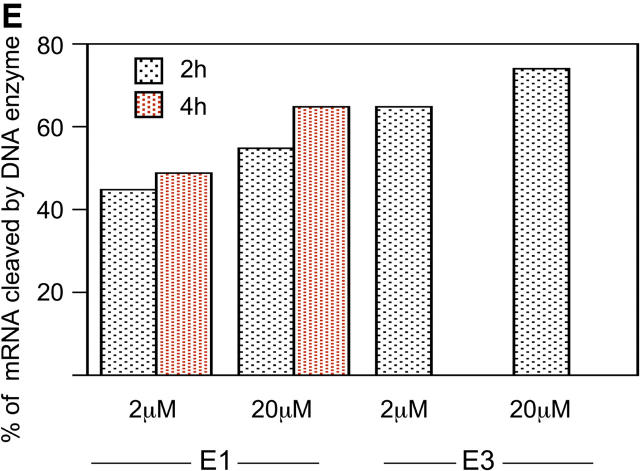
Construction of DNA enzymes and specific cleavage of human PAI-1 mRNA by E1 and E3. (A) Three DNA enzymes were constructed, termed E1, E2, and E3, containing identical 15-nucleotide catalytic domains that were flanked by two arms of eight nucleotides (E1 and E2) or nine nucleotides (E3) with complementarity to human (E1 and E3) or rat (E2) PAI-1 mRNA. To produce the control DNA enzyme E0, the nucleotide sequence in the two flanking arms of E2 was scrambled without altering the catalytic domain. The 3′ terminus of each molecule was capped with an inverted 3′-3′–linked thymidine for resistance to 3′ to 5′ exonuclease digestion. (B) 32P-labeled 21-base oligomer S1, synthesized from human PAI-1 mRNA, was cleaved in a time-dependent manner by E1 at a 10:1 substrate/enzyme excess. (C) Sequence-specific cleavage of human PAI-1 mRNA in vitro generated transcript by DNA enzyme E1 compared with control DNA enzyme E0. In lanes 2 and 4, incubation with E1, but not E0, after preheating transcript to 72°C for 10 min demonstrates further increase in cleavage. (D) Concentration-dependent cleavage of 32P-labeled human PAI-1 mRNA in vitro generated transcripts by E3. The 520 nucleotide transcript was cleaved to products of 320 and 200 nucleotides. (E) Quantitation of representative transcript cleavage by PAI-1 DNA enzymes from two separate experiments, showing time and dose dependence of the cleavage by DNA enzyme.