Figure 5.
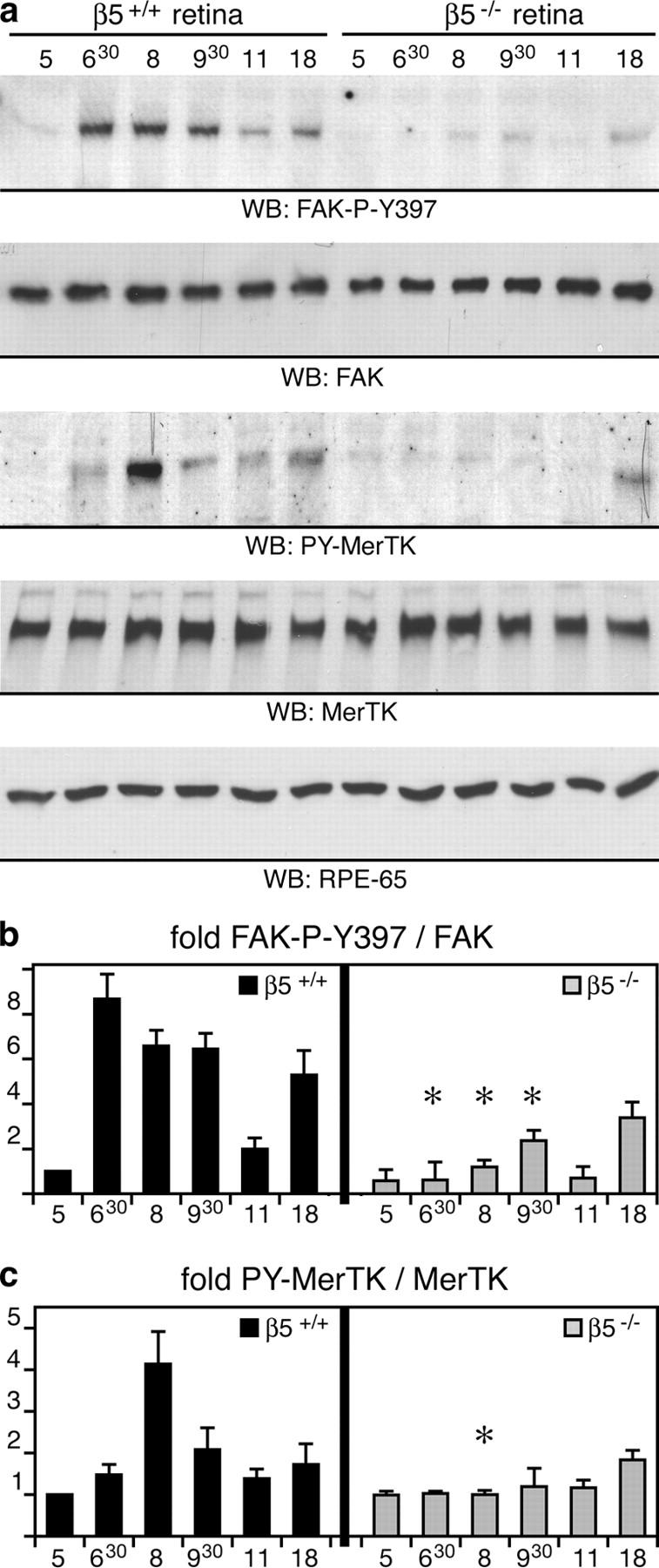
β5 integrin–deficient retina lacks synchronized activation of FAK and MerTK. (a) Eyecup detergent lysates were prepared from 3-wk-old wild-type and β5−/− mice at different times of the day, as indicated. Expression and phosphorylation profiles of proteins were compared by immunoblotting with primary antibodies as indicated in the panels. (b and c) Specific activities of FAK and MerTK were determined as in Fig. 4. Promptly after light onset, levels of active, phosphorylated FAK and MerTK increased in wild-type eyecups. This increase was absent in β5−/− eyecups. Similar results were obtained comparing expression and activity profiles of FAK and MerTK in wild-type and β5−/− mice at 7 and 12 mo (unpublished data). Note that the rise in FAK phosphorylation preceded the increase of MerTK phosphorylation. This agreed well with our earlier data from in vitro phagocytosis assays showing that MerTK activation upon POS challenge requires FAK activation (reference 29). Ratios of active, phosphorylated protein/total protein are given as fold change compared with ratios in wild-type eyes at 5.00 h, which were set at 1.0 arbitrarily. Results are presented as means ± SD. Asterisks indicate significant differences in ratios (n = 3, Student's t test, P < 0.05) between wild-type and β5−/− samples at the same time points.
