Abstract
Four full-thickness skin wounds made in normal mice led to the significant increase in levels of nerve growth factor (NGF) in sera and in wounded skin tissues. Since sialoadenectomy before the wounds inhibited the rise in serum levels of NGF, the NGF may be released from the salivary gland into the blood stream after the wounds. In contrast, the fact that messenger RNA and protein of NGF were detected in newly formed epithelial cells at the edge of the wound and fibroblasts consistent with the granulation tissue produced in the wound space, suggests that NGF was also produced at the wounded skin site. Topical application of NGF into the wounds accelerated the rate of wound healing in normal mice and in healing-impaired diabetic KK/Ta mice. This clinical effect of NGF was evaluated by histological examination; the increases in the degree of reepithelialization, the thickness of the granulation tissue, and the density of extracellular matrix were observed. NGF also increased the breaking strength of healing linear wounds in normal and diabetic mice. These findings suggested that NGF immediately and constitutively released in response to cutaneous injury may contribute to wound healing through broader biological activities, and NGF improved the diabetic impaired response of wound healing.
Repair of wounds is a chain of processes necessary for removal of damaged tissues or invaded pathogens from the body and for complete or incomplete remodeling of injured tissues. The healing process requires a sophisticated interaction between inflammatory cells, biochemical mediators including growth factors, extracellular matrix molecules, and microenvironmental cell populations (1, 2). Inflammatory cells, keratinocytes, and fibroblasts in the wound space and border produce and release a variety of growth factors such as platelet-derived growth factor (PDGF)1, epidermal growth factor (EGF), fibroblast growth factor (FGF), and TGF, which have biological activities to stimulate infiltration of inflammatory cells into the wound space, induce proliferation of keratinocytes and fibroblasts, lead to new formation of capillaries in the granulation tissue, and modulate extracellular matrix deposition and reconstitution of the injured area (1–5). In fact, topical application of some growth factors is successful to accelerate healing of full-thickness wounds in normal mice and normalize a delayed healing response of diabetic mice (6, 7).
Cutaneous wounds often cause anatomical and/or functional damage of peripheral sensory neurons widely distributed in the skin, and nerve growth factor (NGF), which is probably produced in the affected tissue area, may be essential to regenerate the injured neurons. Patients with diabetes mellitus manifest acute and chronic complications including cutaneous infections, immunodisturbance, and vascular and neuropathic dysfunctions (8). Impaired production of NGF has been reported in the submaximal gland of genetically diabetic db/db mice (9) and streptozotocin-induced diabetic mice (10), and in the serum and skin of patients with diabetes mellitus (11, 12). Although NGF is a neurotrophic polypeptide mandatory for the development and function of peripheral and central neurons (13–15), recent findings have shown that NGF regulates immune and inflammatory responses through direct and/or indirect effects on immunocompetent cells (16–22). Biologic actions of NGF are mediated through two types of specific receptors with distinct affinities (23, 24); the low affinity receptor is a 75-kD glycoprotein and the high affinity receptor is a 140-kD molecules with a transmembrane tyrosine kinase domain that is coded by the trk protooncogene (25). We have been studying novel roles of NGF in the processes of inflammation and tissue repair. NGF caused a significant stimulation of granulocyte differentiation from human peripheral blood and murine bone marrow cells (26–28), suppressed apoptosis of rodent neutrophils and peritoneal mast cells (29, 30), enhanced functional properties of murine neutrophils and human eosinophils (20–22), and not only promoted colony formation of murine IL-3–dependent bone marrow–derived cultured mast cells, but also induced the phenotypic change to connective tissue–type mast cells (31).
NGF is produced by many types of cells including fibroblasts (31, 32), keratinocytes (33), mast cells (34), and T cells (35). Therefore, there is a possibility that NGF produced at the wounded site may regulate the healing of the cutaneous wounds. In the present study, we demonstrated that cutaneous wounds resulted in NGF production by the salivary gland and regenerated keratinocytes at the edge of the wound and fibroblasts in the granulation tissue during a wound healing process, and that the topical application of NGF to cutaneous wounds accelerated the rate of wound healing in normal and diabetic mice.
Materials and Methods
Mice.
SJL/J mice were provided from N. Watanabe (Jikei University School of Medicine, Tokyo, Japan). C57BL/6 and genetically diabetic KK/Ta mice were purchased from Clea Japan (Tokyo, Japan). All mice (male, 8–10 wk of age) were kept within a filter-air laminar flow enclosure, and provided with standard laboratory food and water ad libitum. All KK/Ta mice were diagnosed to be diabetic at the beginning of the study.
Cytokines and Other Reagents.
2.5S NGF isolated from murine submaxillary glands was a gift from A.M. Stanisz and J. Bienenstock (McMaster University, Hamilton, Ontario, Canada; reference 26). The preparations were purified by gel filtration on a Sephadex G-75 column to remove traces of renin and IgG sometimes found as contaminants in the original preparation, and further purified by affinity column chromatography with anti– mouse NGF mAb (clone β1). The affinity-purified NGF preparation was eluted as a single protein on an HPLC column (TSK 3,000; Beckman Instruments, Fullerton, CA) with a retention time that corresponded to 27 kD (2.5S NGF dimer; reference 26). Neither EGF activity by an ELISA nor endotoxin activity by a limulus assay was detected, even at a high concentration (10 μg/ml) of the ultrapurified NGF preparation. Neurotrophic activity of the ultrapurified NGF preparation was determined as previously described (31). Recombinant murine IL-1β, IFN-γ, and TNF-α, and recombinant human PDGF B chain (PDGF-BB) homodimer were purchased from Genzyme Corp. (Cambridge, MA). Recombinant human basic FGF (bFGF) was purchased from Upstate Biotechnology Inc. (Lake Placid, NY). EGF purified from murine submaxillary glands was provided by Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Recombinant human TGF-β1 was a gift from H. Akiyama (Kirin Brewery Company, Ltd., Tokyo, Japan). Rabbit anti–mouse 2.5S NGF polyclonal Ab and goat anti–rabbit IgG (H+L) polyclonal Ab conjugated with peroxidase were obtained from Sigma Chemical Co. (St. Louis, MO) and BioMakor (Kirat Weizmann, Rehovot, Israel), respectively. Mouse anti-2.5S NGF mAb was purchased from Boehringer Mannheim GmbH (Mannheim, Germany). All chemicals used were purchased from Sigma Chemical Co., unless otherwise indicated.
Surgical Wounding.
Mice were wounded by using a modification of the technique described by Denon et al. (36). Under pentobarbital sodium anesthesia, hairs on the dorsum of mice were clipped, and four full-thickness round skin wounds (5 mm diam) were prepared using a disposable skin punch equipment (Maruho Co., Ltd., Osaka, Japan). Each wound was separated by at least 1.5 cm of unwounded skin. A group of SJL/J mice was sialoadenectomized or sham operated under pentobarbital sodium anesthesia 4 wk before wounding. Heparinized peripheral blood was collected from the axillary artery at 0, 1, 3, 6, and 24 h after the skin punching. Small pieces of skin samples were removed from the wounds and normal dorsal sites on 0, 1, 3, 7, 10, and 14 d after the skin punching. All the blood and skin samples were obtained under ether anesthesia, and were treated with protease inhibitors (Boehringer Mannheim GmbH) according to the manufacturer's instructions. NGF levels in sera and extracts from the skin tissues were measured by an ELISA using anti-NGF mAb (31), which was sensitive to a lower limit of 50 pg/ml.
Immunohistochemical Examination.
Small pieces were cut from skin tissues with wounds gently smoothed and flattened onto a piece of thick filter, and were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 12 h at 4°C. Skin tissues were embedded in paraffin and cut at 4 μm; the sections were placed on silane-coated glass slides. Tissues were deparaffinized, rehydrated, and washed in PBS (pH 7.4). After pretreatment with a solution of PBS supplemented with 0.3% hydrogen peroxide and 0.1% sodium azide for 10 min to inhibit endogenous peroxidase, the preparations were washed in PBS twice, and then incubated with blocking medium (10% normal goat serum in PBS) for 10 min. Rabbit anti–mouse 2.5S NGF polyclonal Ab diluted 1:2,000 in PBS supplemented with 1% BSA was applied for overnight at 4°C. After washing in PBS twice, peroxidase-conjugated goat anti–rabbit IgG (H+L) Ab diluted 1:100 in PBS was overlaid for 30 min. Visualization of the reaction products was performed with 0.2 mg/ml 3-3′ diaminobenzidine tetrahydrochloride in PBS supplemented with 0.003% hydrogen peroxide. The tissues were counterstained with hematoxylin after the immunoreactions. Thin sections of submaxillary glands were provided as a positive control.
In Situ Hybridization.
A digoxigenin-labeled antisense oligonucleotide primer (5′-AAGGGAATGCTGAAGTTTAGTCCAGTGGGCTTCAGGGACAGAGTCTCC-3′) complementary to nucleotides 378–425 of messenger RNA (mRNA) of mouse NGF (37) was commercially synthesized (Nippon Flour Mills Corp., Tokyo, Japan). Deparaffinized tissue sections were washed in 2× SSC for 10 min at 60°C, rinsed in 0.05M Tris-HCl (pH 7.6), and incubated in 100 μg/ml proteinase K (Nacalai Tesque, Kyoto, Japan) in 0.05 M Tris-HCl (pH 7.6) for 10 min at 37°C. After washing in PBS, the tissue preparations were immersed in 0.4% paraformaldehyde in PBS, pH 7.4, for 10 min at 4°C to arrest proteolytic activity of proteinase K and rinsed in water. To inhibit endogenous alkaline phosphatase activity, the specimens were treated with 0.2 N hydrogen chloride for 10 min. Hybridization was performed using a slight modification of the method reported previously (38). The specimens were hybridized with the digoxigenin-labeled probe (20 ng/ml) in a solution of 50% formamide, 4× SSC, 0.02% Ficoll (type 400), 0.02% polyvinylpyrrolidone, 0.02% BSA, 0.5 μg/ml salmon sperm DNA, 1% sarcosyl (N-lauroyl sarcosine), 10% dextran sulfate, 0.1 M phosphate buffer (pH 7.2), and 0.05 M dithiothreitol, for 16 h at 42°C in a humidified chamber. After washing, the slides were incubated with alkaline phosphatase–conjugated sheep antidigoxigenin Ab (Boehringer Mannheim GmbH), and the reaction products were visualized according to the manufacturer's instructions.
A synthesized sense oligonucleotide primer was used as a negative control. The other control experiments were performed as follows: (a) RNase A treatment before hybridization, and (b) neither a probe nor antidigoxigenin Ab. In these experiments, little or no positive reaction was detected.
Production of NGF by Fibroblasts and Keratinocytes.
The contact-inhibited Swiss albino mouse embryo–derived 3T3 fibroblasts, obtained from the Japanese Cancer Research Resources Bank (Tokyo, Japan), and the transformed BALB/c mouse–derived keratinocytes (PAM 212), provided from I. Katayama (Tokyo Medical and Dental University School of Medicine, Tokyo, Japan), were seeded in 35-mm culture dishes (Nunc, Roskilde, Denmark) at a concentration of 5.0 × 104 cells/ml in 1 ml of α-MEM (GIBCO BRL, Gaithersburg, MD) with 10% FCS (Hyclone Labs, Logan, UT). The culture dishes that contained a confluent monolayer of 3T3 fibroblasts (0.5–1.5 × 106 cells) on 3 d in culture were further incubated at 37°C for 6 d in humidified atmosphere with 5% CO2 in air after the culture medium was replaced with 1 ml of fresh medium containing various concentrations of cytokines or histamine. PAM 212 keratinocytes were incubated with various concentrations of cytokines or histamine for 2 d. The culture medium of both the cells was collected to measure NGF levels (see above) and the number of fibroblasts and keratinocytes was counted in trypsinized cultures.
Treatment of Wound.
Two different types of experiments were conducted to evaluate a stimulating effect of NGF on wound healing in C57BL/6 and genetically diabetic KK/Ta mice (39– 41). First, after skin punching (see above), 20 μl (1 μg) of 2.5S NGF and vehicle solution alone (α-MEM with 10% FCS) were daily applied to two left and two right wounds, respectively. Applications were carried out under pentobarbital sodium anesthesia until the third day, and a healing term was assessed macroscopically. In other experiments, the wounds were removed 8 d after skin punching, and were fixed in phosphate-buffered formalin. Paraffin sections (5 μm thick) were made by routine methods and stained with hematoxylin and eosin. Second, breaking strength of healed linear wounds was examined according to a slight modification of the methods previously reported (6). An anterior-posterior linear incision (4 cm in length) in full thickness was applied to the dorsum of mice with a scalpel under pentobarbital sodium anesthesia. 50 μl (2 μg) of 2.5S NGF or vehicle solution alone were administrated to the incisions, and then the wounds were closed by wound clips placed at 1-cm intervals. Mice were killed 9 d later, and three pieces of skin (0.8 cm in width) between the wound clips were cut vertical to the linear incision. Breaking strength of wounds was measured by using a Rheo meter (NRM-2002J; Fudoh Kogyo Co., Ltd, Tokyo, Japan). The ends of the skin strip were pulled at a constant speed (20 cm/min), and breaking strength was expressed as the mean maximum level of tensile strength (g/mm) before separation of wounds.
Statistical Analysis.
Two-tailed Student's t test was done for statistical analysis of the data and P <0.05 was taken as the level of significance.
Results
NGF Levels in Serum.
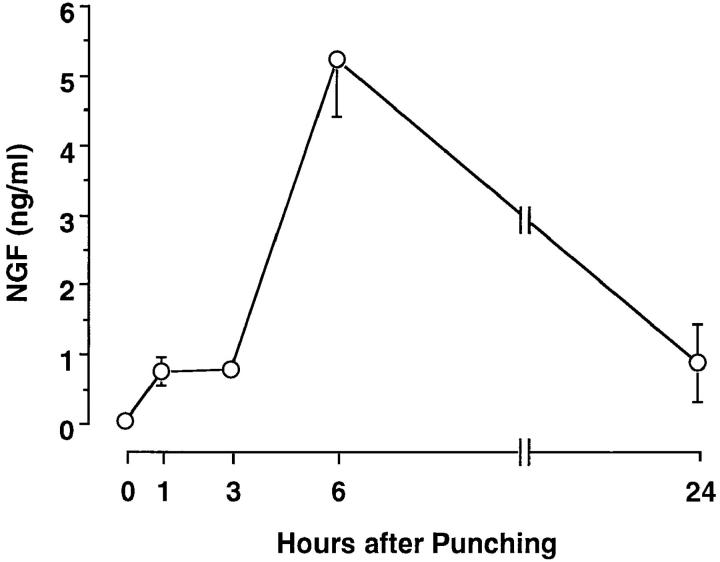
Four full-thickness round wounds were made at the dorsal skin in SJL/J mice, and serum samples were collected under ether anesthesia at various times after the skin punching. Although no NGF was detected in sera isolated from anesthetized mice before the skin punching, the cutaneous wounding resulted in a rapid increase in serum levels of NGF. Serum NGF was at a significant level of 0.72 ng/ml at 1 h, reached a maximal level of ∼5.20 ng/ ml at 6 h, and retained a significant level of ∼0.88 ng/ml even 24 h after the skin punching (Fig. 1). The serum collected at 6 h after the skin punching stimulated the outgrowth of nerve fibers from rat pheochromocytoma cells (PC12); the neurotrophic activity was completely abolished by the addition of anti-NGF Ab (data not shown).
Figure 1.
Serum NGF levels in male SJL/J mice after cutaneous wounding. Each point represents the mean ± SE of three separate experiments using duplicate samples.
NGF Levels in Skin Tissues.
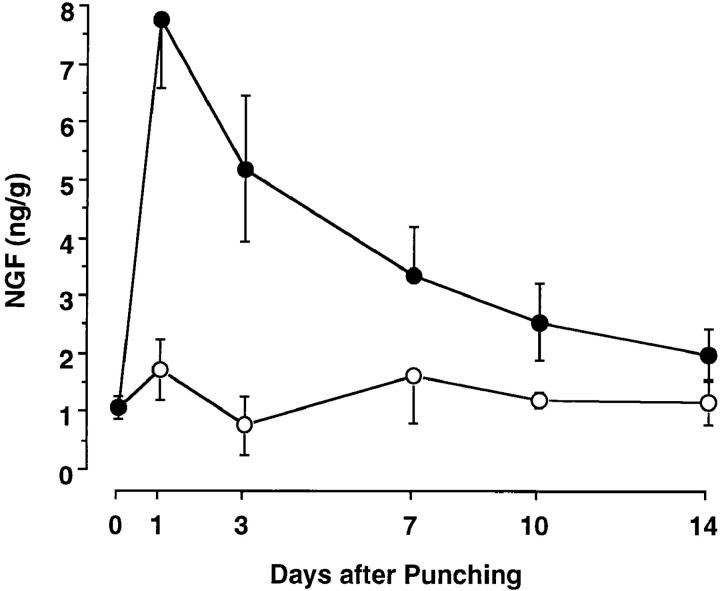
To examine the possible local production of NGF at wounded sites, NGF contents at unwounded and wounded sites were measured on various days after skin punching. All the cutaneous wounds were completely closed by 11 d. Low levels of NGF were detected at uninjured control skin sites isolated on various days after wounding, ranging from 0.81 to 1.7 ng/g. In contrast, at the wounded sites, NGF reached a maximal level of 7.8 ng/g 1 d later, and then its levels were gradually decreased but were higher than those at uninjured control skin sites during the period of 14 d (Fig. 2).
Figure 2.
NGF levels at the wounded skin site (closed) and control unwounded skin site (open) of male SJL/J mice after cutaneous wounding. Each point represents the mean ± SE of three separate experiments using duplicate samples.
Effect of Sialoadenectomy on NGF Levels in Sera and Wounded Skin Sites.
Since the increased NGF in sera after fighting stress has been reported to be originated from the submaxillary glands (42), we conducted some experiments to examine whether sialoadenectomy before cutaneous wounds affected NGF levels in sera and wounded skin sites. Serum NGF levels at 6 h in sialoadenectomized SJL/J mice were lower than the detection limit of an ELISA, but the increased levels were observed in sera of sham-operated mice as roughly comparable to those in nonoperated normal mice (Table 1). On the other hand, sialoadenectomy had no influence on NGF contents at wounded skin sites 3 d after the skin punching. Thus, we concluded that cutaneous wounds led to rapid release of NGF from the submaxillary glands to the peripheral blood and the subsequent local production of NGF at the wounds.
Table 1.
NGF Levels in Sera and Wounded Skin Sites of Sialoadenectomized Mice
| Sialoadenectomy* | NGF concentrations‡ | |||
|---|---|---|---|---|
| Serum | Wounded site | |||
| ng/ml | ng/g | |||
| No | 4.22 ± 0.89 | 4.67 ± 0.11 | ||
| Yes | <0.05 | 3.91 ± 0.46 | ||
SJL/J mice were sialoadenectomized or sham operated 4 wk before skin punching.
Sera and skin tissues including wound were obtained 6 h and 3 d after skin punching, respectively. Each value represents the mean ± SE of five separate experiments in duplicate.
Production of NGF at Wounded Skin Sites.
To identify cell populations that produced NGF at wounded skin sites in SJL/J mice 3 d after skin punching, in situ hybridization analysis and immunohistochemical examination were provided. Granulation tissue including fibroblasts, capillaries, and various kinds of inflammatory cells filled the wound space under crustal tissue. Epithelium at the edge of the wound was several cell layers thick. The leading single cell layer edge of the epithelium was evident over the newly formed granulation tissue, but reepithelialization was only 10–20% at this time. The stratified epithelial cells at the wound edge showed positive staining for mRNA and protein of NGF; deeper layer epithelial cells were strongly positive for its mRNA staining, but superficial layer epithelial cells were strongly positive for its protein staining (Fig. 3, B and F). In addition to the neoepithelium, fibroblasts in granulation tissue formed in the wound space and wound edge were positive for mRNA and protein of NGF (Fig. 3, D and H). In contrast, little or no reaction was observed in epidermal keratinocytes and dermal fibroblasts at the unwounded control skin site. Thus, NGF was produced by stratified epithelial cells and fibroblasts in granulation tissue formed after wounding.
Figure 3.
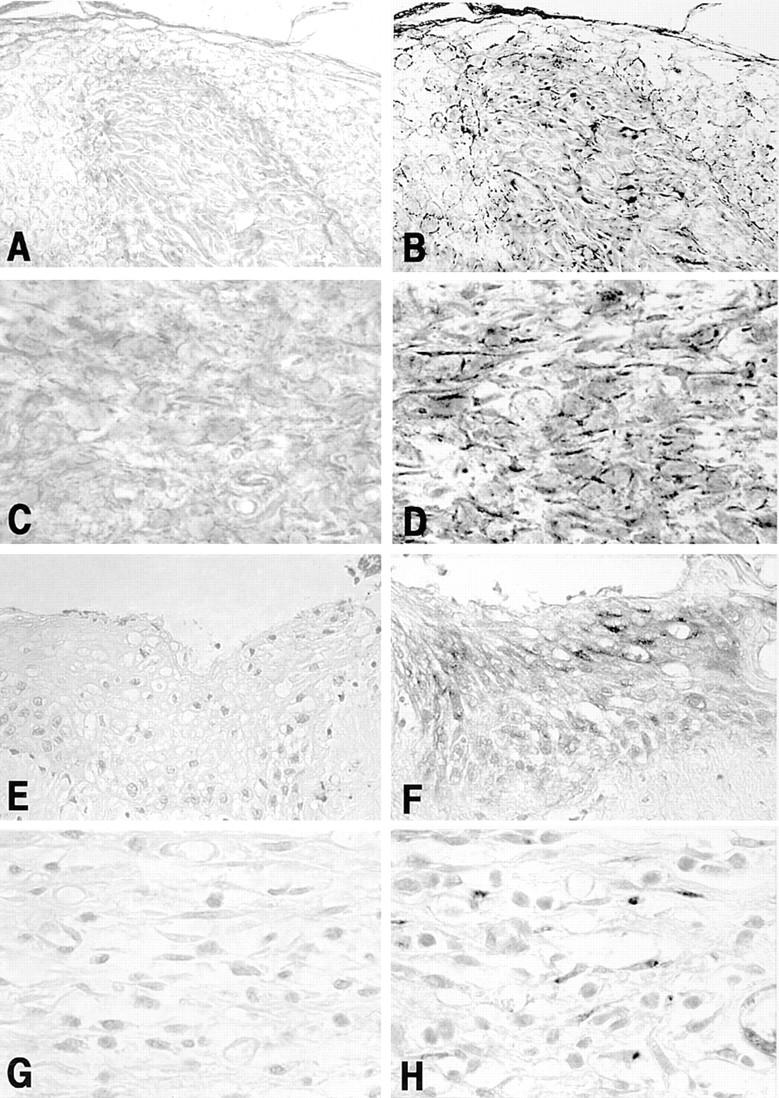
Cellular localization of mRNA and protein of NGF in newly formed epithelium at edge of the wound (B and F, original magnification: 240) and granulation tissue produced in the wound space (D and H, original magnification: 550). All specimens were obtained from male SJL/J mice 3 d after cutaneous wounding. Basal cells of the epidermis show positive reaction for mRNA expression of NGF (B) and superficial epithelial cells show positive reaction for protein of NGF (F). Positive reaction for both the mRNA (D) and protein (H) of NGF is observed in fibroblasts in the granulation tissue. No positive reactions are observed in the sections treated with the sense primer (A and C) and the irrelevant Ab instead of anti-NGF Ab (E and G).
Effect of Various Cytokines and Histamine on NGF Production by Fibroblasts and Keratinocytes.
Since NGF is produced and released from mouse fibroblasts and keratinocytes in vitro (31, 33), we examined the effect of inflammatory cytokines (EGF, bFGF, IFN-γ, IL-1β, PDGF-BB, TGF-β1, and TNF-α) and histamine on NGF production by 3T3 fibroblasts and PAM 212 keratinocytes. NGF levels were assessed by an ELISA in the culture medium collected from 3T3 fibroblasts on 6 d in culture and PAM 212 cells on 2 d in culture. When 3T3 fibroblasts and PAM 212 cells were cultured in medium alone, the collected culture medium contained 88 ± 3 pg/ml and 111 ± 4 pg/ml, respectively. The addition of EGF, bFGF, IFN-γ, IL-1β, PDGF-BB, TGF-β1, or histamine to 3T3 fibroblasts increased NGF levels in the medium at the individual optimal doses by more than three times as compared with medium alone (Fig. 4). In contrast, the addition of TNF-α at doses of 0.2–2 ng/ml resulted in slight enhancement of NGF production (Fig. 4). When PAM 212 cells were cultured with each cytokine or histamine, NGF levels were 1.5–2.5-fold higher than those in medium alone at the individual optimal doses of all the agents (Fig. 5). There was no significant difference in the number of fibroblasts and keratinocytes at the end of the culture between individual groups.
Figure 4.
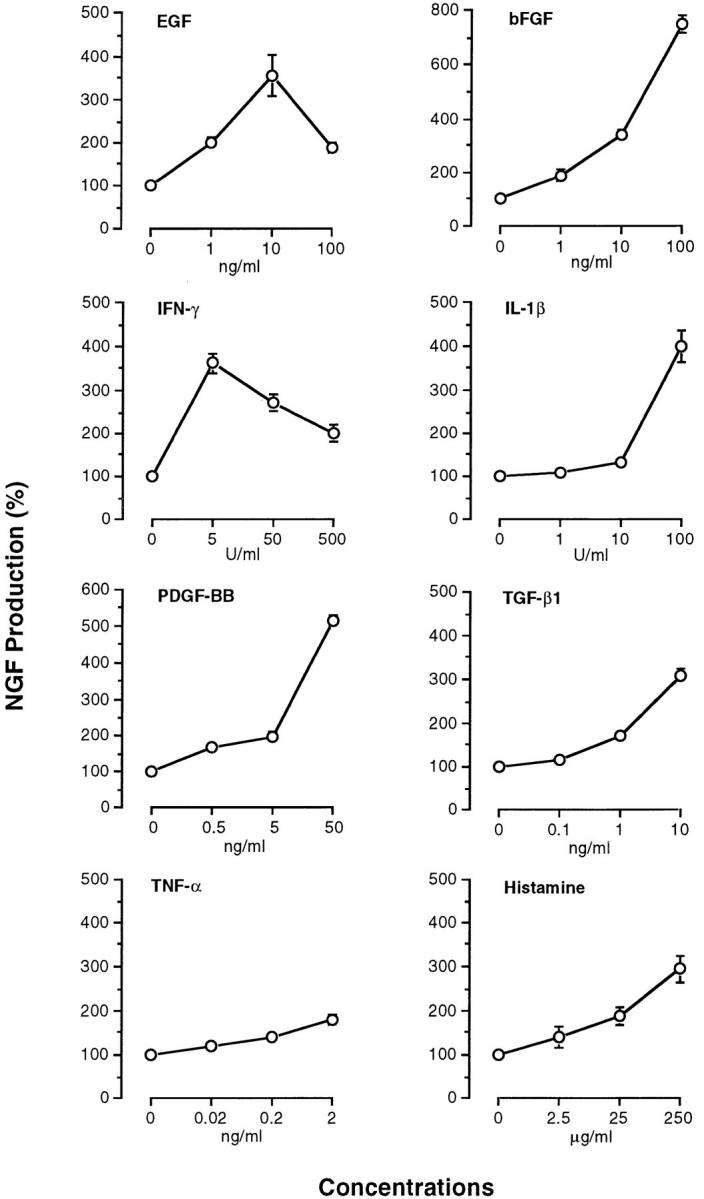
Production of NGF by 3T3 fibroblasts stimulated with various cytokines and histamine. NGF levels in culture medium were measured by a sandwich ELISA as described in Materials and Methods. Each point represents the mean ± SE of three separate experiments using duplicate samples.
Figure 5.
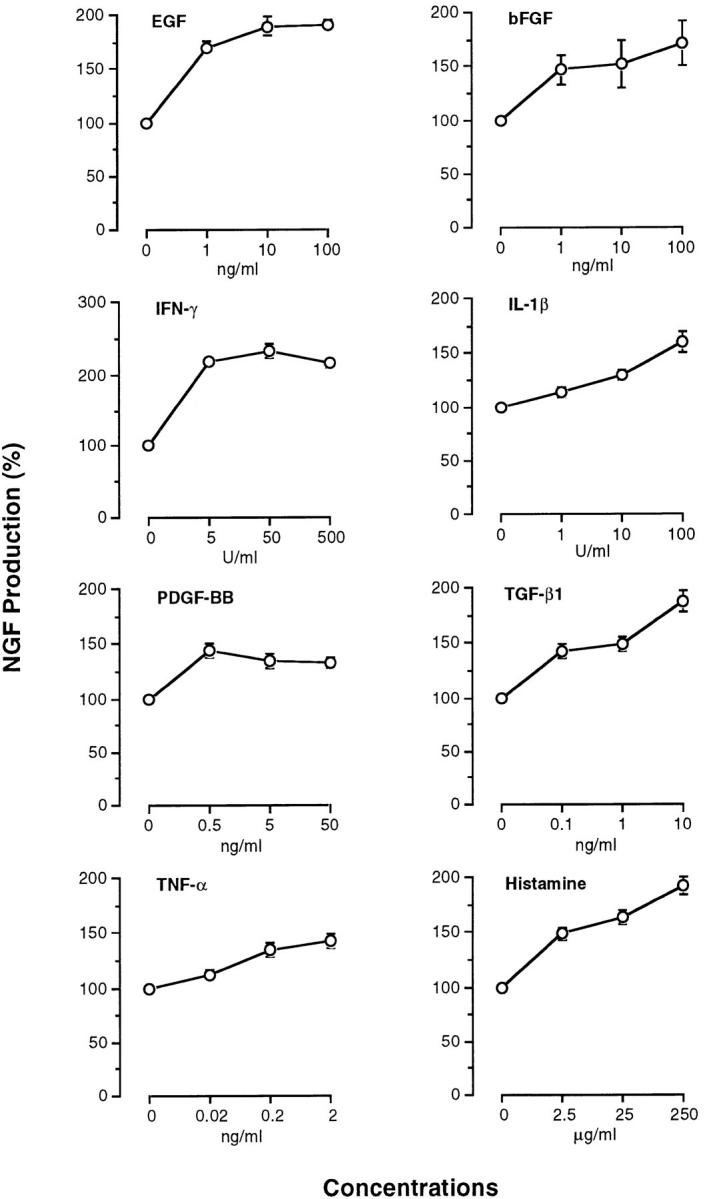
Production of NGF by PAM 212 keratinocytes stimulated with various cytokines and histamine. NGF levels in culture medium were measured by a sandwich ELISA as described in Materials and Methods. Each point represents the mean ± SE of three separate experiments using duplicate samples.
Effect of NGF on the Rate of Wound Healing.
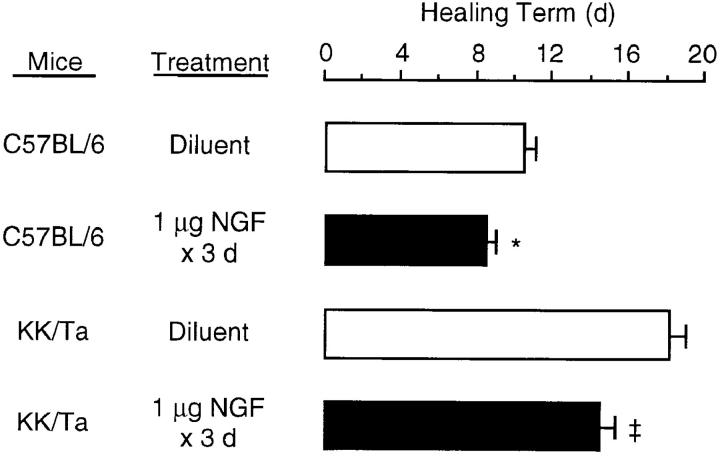
We attempted to assess a possible positive effect of NGF on the rate of cutaneous wound healing in C57BL/6 control mice and genetically diabetic KK/Ta mice. In C57BL/6 mice, right full-thickness round wounds topically treated with only medium alone for 3 d were closed by 11 d (Fig. 6). In KK/ Ta mice, on the other hand, wound closure was delayed >7 d compared to that in C57BL/6 mice, indicating that the rate of wound healing was impaired in KK/Ta mice (P <0.001), the same as genetically diabetic db/db mice (6). When 1 μg NGF was applied to left wounds once per day for 3 d beginning with the day of skin punching, the rate of wound healing was significantly accelerated in both C57BL/6 and KK/Ta mice (Fig. 6). Next, histological examination was performed on the wounds 8 d later. In C57BL/6 mice, topical application of NGF led to the slight accelerating effect on wound healing parameters: complete reepithelialization, an increase in the degree of matrix density, and decreased infiltration of neutrophils (Table 2 and Fig. 7). On the other hand, in KK/Ta mice an impairment in wound healing was evident in incomplete reepithelialization, low deposition of extracellular matrix, and continuous infiltration of numerous neutrophils, but the topical application of NGF improved the parameters of wound healing, which were comparable to those in control C57BL/6 mice without the application of NGF (Table 2 and Fig. 7).
Figure 6.
Wound healing accelerated by topical applications of NGF in control C57BL/6 mice and diabetic KK/Ta mice. NGF (1 μg) or vehicle solution alone was applied to the wound space each day for 3 d beginning with the day of wounding as described in Materials and Methods, and a healing term was examined macroscopically. Each histogram represents the mean ± SE of six mice per group. *P <0.02; ‡ P <0.01; when compared with diluent alone.
Table 2.
Wound Healing Accelerated by Topical Application of NGF in Normal C57BL/6 and Diabetic KK/Ta Mice*
| Mice | NGF | Reepithe- lialization | Thickness of granulation tissue | Matrix density | Acute phase inflam- matory cells | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| C57/BL/6 | No | ++ | ++ | + | + | |||||
| C57/BL/6 | Yes | +++ | ++ | ++ | ± | |||||
| KK/Ta | No | ± | +++ | + | +++ | |||||
| KK/Ta | Yes | + | + | ++ | ++ |
NGF (1 μg) or vehicle solution alone was applied to the wound space each day for 3 d beginning with the day of wounding, and tissue specimens were removed 8 d after the wounding. The wound healing was evaluated by histological parameters indicated. Graded as: −, none; ±, slight; +, mild; ++, moderate; and +++, considerable. Data are representative of four separate experiments.
Figure 7.
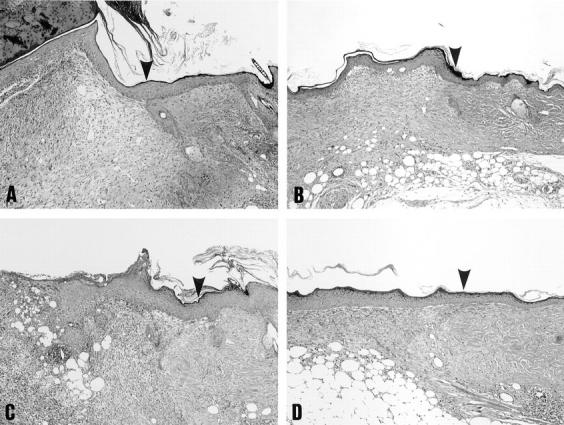
Histological features of wound specimens from control C57BL/6 mice (A and B) and diabetic KK/Ta mice (C and D) 8 d after cutaneous wounding. NGF (1 μg; B and D) or vehicle solution alone (A and C) was applied to the wound space each day for 3 d beginning with the day of wounding as described in Materials and Methods. Sections were stained with hematoxylin and eosin. Original magnification: 60. Arrow heads, the original wound margin.
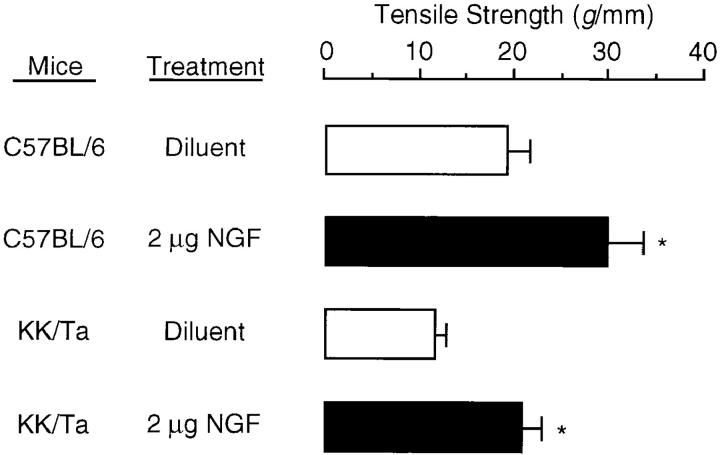
To further evaluate the effect of NGF on wound healing, we attempted to measure breaking strength of anterior–posterior incisional wounds treated with 2 μg NGF or treated with diluent solution alone. Wound specimens were obtained from C57BL/6 and KK/Ta mice 9 d after wounding. Fig. 8 shows that breaking strength of wounds treated with diluent solution alone in C57BL/6 was larger than wounds in KK/Ta mice (P <0.01). A single dose of treatment with NGF was sufficient to induce a significant increase in breaking strength by >1.5 and 2 times in C57BL/6 and KK/Ta mice, respectively. Breaking strength of wounds treated with NGF in KK/Ta mice was comparable to that of wounds treated with diluent solution alone in C57BL/6 mice.
Figure 8.
Effects of NGF on wound tear strength in control C57BL/6 mice and diabetic KK/ Ta mice. NGF (2 μg) or vehicle solution alone was applied to the wound space shortly after the wounding, and wound tear strength was measured as described in Materials and Methods. Each histogram represents the mean ± SE of four mice per group. *P <0.01; when compared with diluent alone.
Specificity of NGF Effects on Wound Healing.
To determine the specificity of the accelerating effects of NGF on wound healing in diabetic KK/Ta mice, NGF was pretreated in vitro with anti-NGF Ab before the topical application to round wounds and linear incisions. As shown in Table 3, NGF pretreated with control Ab induced to accelerate the rate of the wound healing parameters. On the other hand, pretreatment with anti-NGF Ab completely abolished the wound-healing accelerating effects of NGF. Thus, we concluded that topical administration of NGF accelerated the rate of cutaneous wound healing in control normal mice and healing-impaired diabetic mice.
Table 3.
Neutralization of the Wound Healing–accelerating Effect of NGF in Diabetic KK/Ta Mice by Anti-NGF Ab
| Treatment* | Healing term | Tensile strength | ||||
|---|---|---|---|---|---|---|
| Factor | Ab | |||||
| d | g/mm | |||||
| None | None | 18.3 ± 0.4‡ | 10.1 ± 0.8§ | |||
| NGF | Control Ab | 15.0 ± 1.2 | 23.2 ± 2.0 | |||
| NGF | Anti-NGF Ab | 18.5 ± 0.5‡ | 10.3 ± 0.9§ | |||
NGF was mixed with 10-fold diluted rabbit anti–mouse NGF Ab or control Ab at a concentration of 50 μg/ml and incubated at 4°C for 1 h. The NGF mixture was applied to the wound space as described in the legends of Figs. 6 and 8, and then a healing term and wound tear strength were measured. Each value represents the mean ± SE of four separate experiments.
P <0.05; when compared with control Ab.
P <0.001; when compared with control Ab.
Discussion
NGF has the potential to enhance survival and functions of immunocompetent cells, such as neutrophils, eosinophils, mast cells, macrophages, and lymphocytes in rodents (17, 18, 20, 21, 29–31) and humans (19, 22, 43–45), suggesting a possible ability of NGF to promote the rate of cutaneous wound healing. To clarify this point, the present study was conducted. The full-thickness skin wounds at the dorsum of normal mice were able to lead to the rapid increase in levels of NGF in the peripheral blood that possessed a neurotrophic ability. This response was completely abolished by the sialoadenectomy 4 wk before the cutaneous wounds, suggesting that biologically active NGF produced in the submaxillary gland may be released into the peripheral blood. This appears to be consistent with the result of Aloe et al. (42) that showed that aggressive behavior induced the rapid release of NGF from the salivary gland into the blood stream through stimulation of the sympathetic nerve in mice. Thus, biologically active NGF may be released from the salivary gland into the blood stream in response to the cutaneous wound as well as the fighting stress. We also demonstrated that the cutaneous wounds resulted in the significant increase in levels of NGF in the affected cutaneous tissue after the rapid release of NGF into the peripheral blood. Liu et al. (46) reported the increased NGF levels in a wound chamber implanted in axotomized rat sciatic nerve, whereas serum NGF levels remained low. Since the increased level of NGF was not influenced by the sialoadenectomy, and since mRNA and protein of NGF were observed in not only stratified epithelial cells at the edge of the wound and also fibroblasts in the granulation tissue produced in the wound space, the increased NGF in the wounded skin may be mainly produced by newly formed epithelial cells and fibroblasts in the granulation tissue.
A variety of inflammatory cytokines produced by local tissues at the wound acts individually and/or collaboratively in processes of the wound healing and tissue remodeling, and synthesis of the cytokines also seems to be regulated mutually. In fact, IL-1 has a potent ability to upregulate synthesis of NGF in nonneuronal cells of injured rat sciatic nerves (47) and in cultured rat fibroblasts (48). We attempted to demonstrate whether various cytokines (EGF, bFGF, IFN-γ, IL-1β, PDGF-BB, TGF-β1, and TNF-α) and histamine that are produced and released in injured tissues (1, 3–5), affect NGF production by both 3T3 fibroblasts and PAM 212 keratinocytes in vitro. Interestingly, all of the reagents except for TNF-α enhanced NGF production of both of the cell lines. Since little or no reaction for mRNA and protein of NGF was detected in epidermal keratinocytes and dermal fibroblasts at the uninjured control skin site, NGF synthesis in both the cell populations activated and proliferated after the wounds seems to be regulated by such cytokines released from various cell components in local tissues including infiltrating inflammatory cells.
The local application of NGF into cutaneous wounds was sufficient to accelerate the rate of wound healing in normal mice and normalize the delayed healing response in diabetic KK/Ta mice. In addition to the results of healing rate and breaking strength of wounds, the histological findings, such as the increases in the degree of reepithelialization, the thickness of the granulation tissue, and the density of extracellular matrix, provided distinct evidence that NGF had a biological ability to improve the degree of the parameters of wound healing in normal mice and even in healing-impaired diabetic mice. NGF also modulates proliferation of keratinocytes in mice (49) and humans (50) through the high affinity receptor, suggesting that NGF produced from newly formed epithelial cells at the edge of the wound may support reepithelialization through autocrine stimulation mechanisms involving synergy with the other cytokines. In contrast, since murine 3T3 fibroblasts have no expression of the NGFR on their surface (21), the granulation tissue and matrix formation induced after NGF application might be caused by indirect promoting effects through cytokines such as bFGF, PDGF, and TGF-β1, which contribute to proliferation of fibroblasts and synthesis of extracellular matrix by fibroblasts (51–53).
Peripheral neuropathy that occurs by complicated metabolic mechanisms is one of the common complications distressing patients with diabetes mellitus; impairment of sensory neurons presents as pain and loss of sensation, and often results in cutaneous infection and impaired wound healing (8). The recent report (13) demonstrating the decreased levels of endogenous NGF, a sensory neurotrophic factor, and substance P, a sensory neurotransmitter, in the skin of patients with diabetes mellitus, gives rise to a possibility that impairment of NGF production may also contribute to the pathogenesis of diabetic peripheral neuropathy. In fact, diabetic KK/Ta mice showed impaired regeneration of nerve fibers after wounding (data not shown), and the topical administration of NGF significantly accelerated the regeneration of nerve fibers that were roughly comparable to that of control C57BL/6 mice. Apfel et al. (54) demonstrated that exogenously administered NGF was capable of preventing the behavioral and biochemical manifestations of sensory neuropathy in streptozotocin-induced diabetic rats. The present study clearly demonstrated that the topical administration of NGF into the full-thickness wounds by skin punching normalized the defect of diabetic KK/Ta mice regarding the rate of wound healing. Thus, we consider that NGF has a potentiality as a therapeutic agent for the normalization of the diabetic impaired response of wound healing.
Acknowledgments
We would like to thank Drs. J. Bienenstock and A.M. Stanisz (McMaster University, Hamilton, Ontario, Canada) for providing ultrapurified NGF, Dr. I. Katayama (Tokyo Medical and Dental University, Tokyo, Japan) for providing PAM 212 keratinocytes, and Dr. H. Akiyama (Kirin Brewery Company, Ltd., Tokyo, Japan) for supplying TGF-β1. We also thank Dr. R. Tsuboi (Juntendo University, Tokyo, Japan) for helpful discussions.
This work was supported by grants from the Ministry of Education, Science, Sports, and Culture, Japan, for Recombinant Cytokine's Project provided by the Ministry of Agriculture, Forestry, and Fisheries, Japan (RCP 1998-4120), and from Lydia O'Leary Memorial Foundation.
Footnotes
Abbreviations used in this paper: bFGF, basic FGF; EGF, epidermal growth factor; FGF, fibroblast growth factor; mRNA, messenger RNA; NGF, nerve growth factor; PDGF, platelet-derived growth factor.
References
- 1.Clark, R.A.F. 1996. Wound repair: overview and general considerations. In The Molecular and Cellular Biology of Wound Repair. R.A.F. Clark, editor. Plenum Press, New York. 3–50.
- 2.Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 3.Pierce GF, Mustoe TA. Pharmacologic enhancement of wound healing. Annu Rev Med. 1995;46:467–481. doi: 10.1146/annurev.med.46.1.467. [DOI] [PubMed] [Google Scholar]
- 4.Bennett NT, Schultz GS. Growth factors and wound healing: biochemical properties of growth factors and their receptors. Am J Surg. 1993;165:728–737. doi: 10.1016/s0002-9610(05)80797-4. [DOI] [PubMed] [Google Scholar]
- 5.Moulin V. Growth factors in skin wound healing. Eur J Cell Biol. 1995;68:1–7. [PubMed] [Google Scholar]
- 6.Tsuboi R, Rifkin DB. Recombinant basic fibroblast growth factor stimulates wound healing-impaired db/dbmice. J Exp Med. 1991;172:245–251. doi: 10.1084/jem.172.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown RE, Breeden MP, Greenhalgh DG. PDGF and TGF-alpha act synergistically to improve wound healing in the genetically diabetic mouse. J Surg Res. 1994;56:562–570. doi: 10.1006/jsre.1994.1090. [DOI] [PubMed] [Google Scholar]
- 8.Siegel, J. 1995. Diabetes mellitus. In Perspectives on Pathophysiology. L.-E.C. Copstead, editor. W.B. Saunders Company, Philadelphia. 826–845.
- 9.Kasayama S, Oka T. Impaired production of nerve growth factor in the submandibular gland of diabetic mice. Am J Physiol. 1989;257:E400–E404. doi: 10.1152/ajpendo.1989.257.3.E400. [DOI] [PubMed] [Google Scholar]
- 10.Ordonez G, Fernandez A, Perez R, Sotelo J. Low contents of nerve growth factor in serum and submaxillary gland of diabetic mice. A possible etiological element of diabetic neuropathy. J Neurol Sci. 1994;121:163–166. doi: 10.1016/0022-510x(94)90346-8. [DOI] [PubMed] [Google Scholar]
- 11.Faradji V, Sotelo J. Low serum levels of nerve growth factor in diabetic neuropathy. Acta Neurol Scand. 1990;81:402–406. doi: 10.1111/j.1600-0404.1990.tb00984.x. [DOI] [PubMed] [Google Scholar]
- 12.Anand P. Neurotrophins and peripheral neuropathy. Philos Trans R Soc Lond B Biol Sci. 1996;351:449–454. doi: 10.1098/rstb.1996.0041. [DOI] [PubMed] [Google Scholar]
- 13.Levi-Montalcini R, Angeletti PU. Essential role of the nerve growth factor on the survival and maintenance of dissociated sensory and sympathetic embryonic nerve cells in vitro. Dev Biol. 1963;7:653–659. doi: 10.1016/0012-1606(63)90149-0. [DOI] [PubMed] [Google Scholar]
- 14.Thoenen H, Edgar D. Neurotrophic factors. Science. 1985;229:238–242. doi: 10.1126/science.2409599. [DOI] [PubMed] [Google Scholar]
- 15.Korsching S. The role of nerve growth factor in the CNS. Trends Neurosci. 1986;9:570–577. [Google Scholar]
- 16.Gee AP, Boyle MDP, Munger L, Lawman MJP, Young M. Nerve growth factor: stimulation of polymorphonuclear leukocyte chemotaxis in vitro. Proc Natl Acad Sci USA. 1983;80:7215–7218. doi: 10.1073/pnas.80.23.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearce FL, Thompson HL. Some characteristics of histamine secretion from rat peritoneal mast cells stimulated with nerve growth factor. J Physiol (Lond) 1986;372:379–393. doi: 10.1113/jphysiol.1986.sp016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorpe LW, Perez-Polo JR. The influence of nerve growth factor on the in vitro proliferative response of rat spleen lymphocytes. J Neurosci Res. 1987;18:134–139. doi: 10.1002/jnr.490180120. [DOI] [PubMed] [Google Scholar]
- 19.Otten U, Ehrhard P, Peck R. Nerve growth factor induces growth and differentiation of human B lymphocytes. Proc Natl Acad Sci USA. 1989;86:10059–10063. doi: 10.1073/pnas.86.24.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kannan Y, Ushio H, Koyama H, Okada M, Oikawa M, Yoshihara T, Kaneko M, Matsuda H. 2.5S nerve growth factor enhances survival, phagocytosis, and superoxide production of murine neutrophils. Blood. 1991;77:1320–1325. [PubMed] [Google Scholar]
- 21.Susaki Y, Shimizu S, Katakura K, Watanabe N, Kawamoto K, Matsumoto M, Tsudzuki M, Furusaka T, Kitamura Y, Matsuda H. Functional properties of murine macrophages promoted by nerve growth factor. Blood. 1996;88:4630–4637. [PubMed] [Google Scholar]
- 22.Hamada A, Watanabe N, Ohtomo H, Matsuda H. Nerve growth factor enhances survival and cytotoxic activity of human eosinophils. Br J Haematol. 1996;93:299–302. doi: 10.1046/j.1365-2141.1996.5151055.x. [DOI] [PubMed] [Google Scholar]
- 23.Sutter A, Riopelle RJ, Harris-Warrick RM, Shooter EM. Nerve growth factor receptors: characterization of two distinct classes of binding sites on chick embryo sensory ganglia cells. J Biol Chem. 1979;254:4972–4982. [PubMed] [Google Scholar]
- 24.Yanker BA, Shooter EM. The biology and mechanism of action of nerve growth factor. Annu Rev Biochem. 1982;51:845–868. doi: 10.1146/annurev.bi.51.070182.004213. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan DR, Hempstead BL, Martin-Zanca D, Chao MV, Parada LF. The trkproto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 1991;252:554–558. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- 26.Matsuda H, Coughlin MD, Bienenstock J, Denburg JA. Nerve growth factor promotes human hemopoietic colony growth and differentiation. Proc Natl Acad Sci USA. 1988;85:6508–6512. doi: 10.1073/pnas.85.17.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuda H, Switzer J, Coughlin MD, Bienenstock J, Denburg JA. Human basophilic cell differentiation promoted by 2.5S nerve growth factor. Int Arch Allergy Immunol. 1988;86:453–457. doi: 10.1159/000234634. [DOI] [PubMed] [Google Scholar]
- 28.Kannan Y, Matsuda H, Ushio H, Kawamoto K, Shimada Y. Murine granulocyte-macrophage and mast cell colony formation promoted by nerve growth factor. Int Arch Allergy Immunol. 1993;132:362–367. doi: 10.1159/000236584. [DOI] [PubMed] [Google Scholar]
- 29.Kannan Y, Usami K, Okada M, Shimizu S, Matsuda H. Nerve growth factor suppresses apoptosis of murine neutrophils. Biochem Biophys Res Commun. 1992;186:1050–1056. doi: 10.1016/0006-291x(92)90853-d. [DOI] [PubMed] [Google Scholar]
- 30.Kawamoto K, Okada T, Kannan Y, Ushio H, Matsumoto M, Matsuda H. Nerve growth factor prevents apoptosis of rat peritoneal mast cells through the trkproto-oncogene receptor. Blood. 1995;86:4638–4644. [PubMed] [Google Scholar]
- 31.Matsuda H, Kannan Y, Ushio H, Kiso Y, Kanemoto T, Suzuki H, Kitamura Y. Nerve growth factor induces development of connective tissue–type mast cells in vitro from murine bone marrow cells. J Exp Med. 1991;174:7–14. doi: 10.1084/jem.174.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young M, Ofer J, Blanchard MH, Asdourian H, Amos H, Arnason BGW. Secretion of a nerve growth factor by primary chick fibroblast cultures. Science. 1974;187:361–362. doi: 10.1126/science.1167427. [DOI] [PubMed] [Google Scholar]
- 33.Tron VA, Coughlin MD, Jang DE, Stanisz J, Sauder DN. Expression and modulation of nerve growth factor in murine keratinocytes (PAM 212) J Clin Invest. 1990;85:1085–1089. doi: 10.1172/JCI114539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leon, A., A. Buriani, R. dal Toso, M. Fabris, S. Romanello, L. Aloe, and R. Levi-Montalcini. 1994. Mast cells synthesize, store, and release nerve growth factor. Proc. Natl. Acad. Sci. USA. 91:3739–3743. [DOI] [PMC free article] [PubMed]
- 35.Ehrhard PB, Erb P, Graumann U, Otten U. Expression of nerve growth factor and nerve growth factor receptor tyrosine kinase Trk in activated CD4-positive T-cell clones. Proc Natl Acad Sci USA. 1993;90:10984–10988. doi: 10.1073/pnas.90.23.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denon D, Kowatch MA, Roth GS. Production of wound repair in old mice by local injection of macrophages. Proc Natl Acad Sci USA. 1989;86:2018–2020. doi: 10.1073/pnas.86.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott J, Selby M, Uredea M, Quiriga M, Bell GI, Rutter WJ. Isolation and nucleotide sequence of cDNA encoding the precursor of mouse nerve growth factor. Nature. 1983;302:538–540. doi: 10.1038/302538a0. [DOI] [PubMed] [Google Scholar]
- 38.Humpel C, Lindqvidst E, Olson L. Detection of nerve growth factor mRNA in rodent salivary glands with digoxigenin- and 32P-labeled oligonucleotides: effects of castration and sympathectomy. J Histochem Cytochem. 1993;41:703–708. doi: 10.1177/41.5.8468451. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura M, Yamada K. Studies on a diabetic (KK) strain of the mouse. Diabetologia. 1967;3:212–221. doi: 10.1007/BF01222198. [DOI] [PubMed] [Google Scholar]
- 40.Reddi AS, Camerini-Davalos RA. Hereditary diabetes in the KK mouse: an overview. Adv Exp Med Biol. 1988;246:7–15. doi: 10.1007/978-1-4684-5616-5_2. [DOI] [PubMed] [Google Scholar]
- 41.Hasegawa M, Ogawa Y, Katsuura G, Shintaku H, Hosoda K, Nakano K. Regulation of obese gene expression in KK mice and congenic lethal yellow obese KKAy mice. Am J Physiol. 1996;271:E333–E339. doi: 10.1152/ajpendo.1996.271.2.E333. [DOI] [PubMed] [Google Scholar]
- 42.Aloe L, Alleva E, Bohn A, Levi-Montalcini R. Aggressive behavior induces release of nerve growth factor from mouse salivary gland into the bloodstream. Proc Natl Acad Sci USA. 1986;83:6184–6187. doi: 10.1073/pnas.83.16.6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ehrhard PB, Ganter U, Bauer J, Otten U. Expression of functional trkprotooncogene in human monocytes. Proc Natl Acad Sci USA. 1993;90:5423–5427. doi: 10.1073/pnas.90.12.5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bischoff S, Dahinden CA. Effect of nerve growth factor on the release of inflammatory mediators by mature human basophils. Blood. 1992;792:2662–2669. [PubMed] [Google Scholar]
- 45.Torcia M, Bracci-Laudiero L, Lusibello M, Nencioni L, Labardi D, Rubartelli A, Cozzolino F, Aloe L, Garaci E. Nerve growth factor is an autocrine survival factor for memory B lymphocytes. Cell. 1996;85:345–356. doi: 10.1016/s0092-8674(00)81113-7. [DOI] [PubMed] [Google Scholar]
- 46.Liu HM, Lei HY, Kao KP. Correlation between NGF levels in wound chamber fluid and cytological location of NGF and NGF receptor in axotomized rat sciatic nerve. Exp Neurol. 1995;132:24–32. doi: 10.1016/0014-4886(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 47.Lindholm D, Heumann R, Meyer M, Thoenen H. Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature. 1987;330:658–659. doi: 10.1038/330658a0. [DOI] [PubMed] [Google Scholar]
- 48.Lindholm D, Heumann R, Hengerer B, Thoenen H. Interleukin 1 increases stability and transcription of mRNA encoding nerve growth factor in cultured rat fibroblasts. J Biol Chem. 1988;263:16348–16351. [PubMed] [Google Scholar]
- 49.Paus R, Luftle M, Czarnetzki BM. Nerve growth factor modulates keratinocyte proliferation in murine skin organ culture. Br J Dermatol. 1994;130:174–180. doi: 10.1111/j.1365-2133.1994.tb02896.x. [DOI] [PubMed] [Google Scholar]
- 50.Di Marco E, Mathor M, Bondanza S, Cutuli N, Marchisio PC, Cancedda R, De Luca M. Nerve growth factor binds to normal human keratinocytes through high and low affinity receptors and stimulates their growth by a novel autocrine loop. J Biol Chem. 1993;268:22838–22846. [PubMed] [Google Scholar]
- 51.Buckley-Sturrock A, Woodward SC, Senior RM, Griffin GL, Klagsbrun M, Davidson JM. Differential stimulation of collagenase and chemotactic activity in fibroblasts derived from rat wound repair tissue and human skin by growth factors. J Cell Physiol. 1989;138:70–78. doi: 10.1002/jcp.1041380111. [DOI] [PubMed] [Google Scholar]
- 52.Pierce GF, Mustoe TA, Altrock BW, Deuel TF, Thomason A. Role of platelet-derived growth factor in wound healing. J Cell Biochem. 1991;45:319–326. doi: 10.1002/jcb.240450403. [DOI] [PubMed] [Google Scholar]
- 53.Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Flalnga V, Kehrl JH, Fauci AS. Transforming growth factor type B: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. . Proc Natl Acad Sci USA. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Apfel SC, Arezzo JC, Brownlee M, Federoff H, Kessler JA. Nerve growth factor administration protects against experimental diabetic sensory neuropathy. Brain Res. 1994;634:7–12. doi: 10.1016/0006-8993(94)90252-6. [DOI] [PubMed] [Google Scholar]