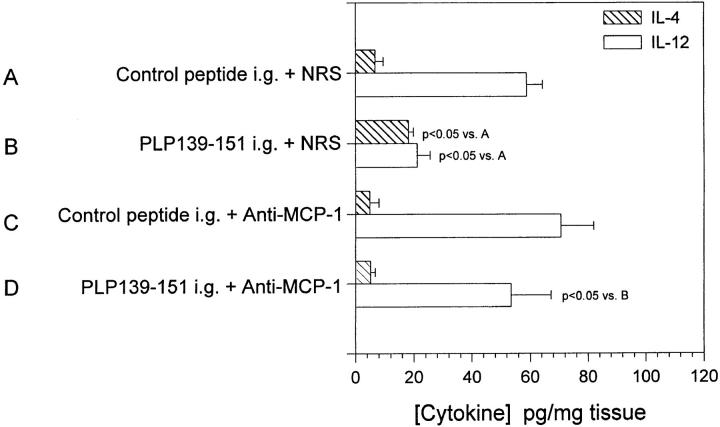
Figure 6.
In vivo anti–MCP-1 treatment abrogates induction of oral tolerance (A) Mice were fed either PLP139–151 to induce immunologic tolerance of control peptide 7 d before disease induction with PLP139– 151 in CFA. Each of those two groups were further divided and treated with either 0.5 ml anti–MCP-1 or NRS 2 d before antigen feeding, the day of antigen feeding, and 2 d after antigen feeding. (B) Mice were fed either PLP139–151 or control peptide 7 d before disease induction with PLP139–151 in CFA. Each of those two groups was further divided and treated with either 0.5 ml anti–MCP-1 or NRS 3, 5, and 7 d after antigen feeding. All animals were followed for the development of clinical EAE according to the grading scale in the Materials and Methods. The data represent the mean clinical disease score in each group and are representative of at least two identical experiments. Animals that were fed PLP139– 151 and treated with NRS (closed circles) developed significantly decreased clinical disease compared to control peptide–fed and NRS-treated mice (closed squares), P < 0.05 days 17–25 after immunization.